3. Требования, предъявляемые к осадкам и весовым формам в гравиметрии.
4. Какие два вида соосаждения существуют в кристаллических осадках?
5. Дайте определение понятиям: инклюзия и окклюзия.
6. Что понимают под чистотой аморфных осадков?
7. Посуда и оборудование, которые применяются в гравиметрическом анализе?
Примеры решения типовых задач
Задача 1. Рассчитайте массу навески квасцов КАI(SO4)2×12H2O, которую следует взять для определения алюминия в виде оксида алюминия, и требующийся для этого объем осадителя.
Решение:
Один из известных способов гравиметрического определения ионов алюминия в его растворимых солях заключается в осаждении осадка Al(ОН)3 при взаимодействии с раствором аммиака с последующим прокаливанием Al(OH)3 и Al2O3.
В данном случае KAl(SO4)2×12H2O – анализируемое соединение, ОВ;
NH3×H2O(NH4OH)- осадитель, РВ;
Al(OH)3 – осаждаемая форма;
Al2O3 - весовая форма;
Al – определяемый элемент.
1. Записываем уравнения химических реакций, протекающих при этом:
Al3+ + 3ОН-Al(OH)3
t
2 Al(OH)3Al2O3 + 3Н2О, то есть
| 6NH4OH |
| t |
KAl(SO4)2×12H2O 2Al(OH)3 Al2O3
2. Поскольку осадок Al2O3 является аморфным, то массу навески рассчитываем по формуле (1), где m(ВФ) = 0,1 г.
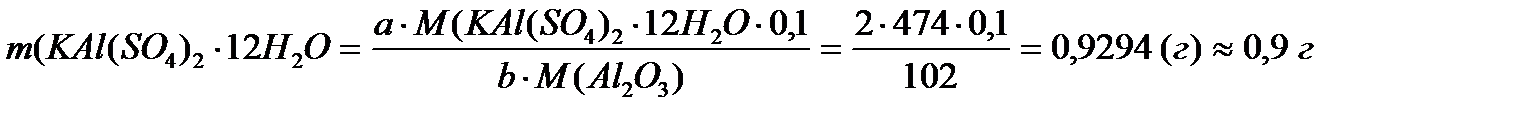
M(KАl(SO4)2×12H2O)=474 г/моль,
М(Al2O3)=102 г/моль,
А=2, b=1.
3. Рассчитываем объем осадителя по формуле (2). В данном случае осадителем является NH3×H2O (NH4OH). Для получения аморфных осадков используют концентрированный раствор: обычно w(NH4OH)=25%;

Ответ: 0,1 г; 1,3 см3
Варианты контрольных заданий
Вариант 1
1.Последовательность аналитических операций в гравиметрическом методе осаждения.
2. Перечислитеосновныетребованиякосадку.Отличиеосаждаемойформыотвесовой.Сколькомл0,5нраствораоксалатааммонияпотребуетсядляосажденияионакальцияизраствора,полученногоприрастворении0,7гкарбонатакальция?
Вариант 2
Механизм образования осадка и условия осаждения.
2. ДляколичественногоопределенияионабариярастворилинавескуBaCl2∙2H2Oв0.4526г.Какойобъем2нрастворасернойкислотыпотребуетсядляполногоосажденияионабария?
Вариант 3
1. Требования, предъявляемые к осадкам и весовым формам в гравиметрии.
2. ОпределитесодержаниебариявобразцехимическичистогоBaCl2∙2H2O,еслинавескачистогоBaCl2∙2H2Oравна0,4872г.Массаосадкасульфатабарияпослепрокаливаниясоставила0,4644г.
Вариант 4
1. Весовойанализ.Основныеоперациивесовогоанализа.Требования,предъявляемыекосадкам(аморфнымикристаллическим).
2. Сколькомл1нрастворахлоридабарияпотребуетсядляосаждениясульфат-иона,еслирастворено2гмедногокупороса,содержащего5%примесей?Учтитеизбытокосадителя.
Вариант 5
1.Гравиметрическийанализ.Чтоназывается«навеской»?Чемопределяетсявыборвеличинынавескианализируемоговещества?
2. Нааналитическихвесахотвесилинавескув0,7178гх.ч.хлоридакальция.Приготовилиизнее250млраствора.Определитеэквивалентнуюконцентрациюититрданногораствора.
Вариант 6
1. Определениесодержанияразличныхвеществ(примесей)илиэлементоввсельскохозяйственныхобъектах.
2. Приопределениижелезавесовымметодомиз1,5гвеществабылополучено0,48гFe2O3.Чемуравнамассоваядоляжелезавобразце?
Вариант 7
1. Опишитеопределениекристаллизационнойводывмедномкупоросегравиметрическимметодом.
2. Приопределениижелезавесовымметодомиз2,5гвеществабылополучено1,48гFe2O3.Чемуравнамассоваядоляжелезавобразце?
Вариант 8
1. Гравиметрия.Преимуществаинедостаткиметода.
2. Прианализе0,8105гсплаваполучено0,5008гAl2O3.Определитепроцентноесодержаниеалюминиявсплаве.
Вариант 9
1. Что понимают под чистотой аморфных осадков?
2. Какуюнавеску сульфатажелезаFeSO4∙7H2Oследуетвзятьдляопределения внем железа ввидеFe2O3(считая нормуосадка равнойпримерно0,2г).
Вариант 10
1. Весовойанализ.Перечислитеусловияосаждениякристаллическихиаморфныхвеществ.Чтотакоеформаосажденияивесовая(гравиметрическая)форма?
2. Какуюнавеску сульфатажелезаFeSO4∙7H2Oследуетвзятьдляопределения внем железа ввидеFe2O3(считая нормуосадка равнойпримерно0,2г).
Вариант 11
1. Характеристикагравиметрическогоанализа.Чтоназывается«навеской»?Чемопределяетсявыборвеличинынавескианализируемоговещества?
2. Дляанализавзято0,5850гхлоридабария.Приегообработкесернойкислотойобразовалсяосадоксульфатабариявколичестве0,5642г.Сколькограммовбариявходитвсоставосадка?Определитьпроцентноесодержаниебариявовзятойнавеске.
Вариант 12
1. Аналитическиеопределениявесовымметодом.Чтоназывается«навеской»?Чемопределяетсявыборвеличинынавескианализируемоговещества?Основныеоперациивесовогоанализа.
2. Нааналитическихвесахотвесилинавескув0,5168гх.ч.хлоридакальция.Приготовилиизнее250млраствора.Определитеэквивалентнуюконцентрациюититрданногораствора.
Вариант 13
1. Операции гравиметрическогоанализа.Требованиякосадкуиосадителю.
2. ДляколичественногоопределенияионабариярастворилинавескуBaCl2∙2H2Oв0.5241г.Какойобъем1,5нрастворасернойкислотыпотребуетсядляполногоосажденияионабария?
Вариант 14
1. Определенияметодомгравиметрии.Перечислитеусловияосаждениякри-сталлическихиаморфныхвеществ.Чтотакоеформаосажденияивесовая(гравиметрическая)форма?Какимтребованиямонидолжныотвечать?
2. ОпределитесодержаниебариявобразцехимическичистогоBaCl2∙2H2O,еслинавескачистогоBaCl2∙2H2Oравна0,7878г.Массаосадкасульфатабарияпослепрокаливаниясоставила0,5654г.
Вариант 15
1. Требования, предъявляемые к осаждаемой и гравиметрической формам.
2. Какойобъем0,8нрастворахлоридабарияпотребуетсядляосаждениясульфат-иона,еслирастворено2,4гмедногокупороса,содержащего10%примесей?
Вариант 16
1. Что такое индукционный период в методе осаждения?
2. Какойобъем0,15нрастворасернойкислотыпотребуетсядляосажденияионовбарияизнавескинитратабариявеличиной0,55г?
Вариант 17
1. Какие два вида соосаждения существуют в кристаллических осадках?
2. Дляанализавзято0,3850гхлоридабария.Приегообработкесернойкислотойобразовалсяосадоксульфатабариявколичестве0,2642г.Сколькограммовбариявходитвсоставосадка?Определитьпроцентноесодержаниебариявовзятойнавеске.
Вариант 18
1. Перечислитеусловияосаждениякристаллическихиаморфныхвеществ,нормыихдляанализа.Что такоеформаосажденияивесовая(гравиметрическая)форма?
2. Дляопределениясодержаниясульфатакалиягравиметрическимметодомиз3,5гобразца,содержащегосульфаткалия,былополучено3,12гсульфатабария.Определитемассовуюдолюсульфатакалиявобразце.
Вариант 19
1. Укажите оптимальные условия осаждения аморфных осадков.
2. Опишите гравиметрическое определение магния в хлориде магния в виде MgNH4PO4×6H2O. Укажите определяемое вещество, осадитель, осаждаемую и весовую формы. Рассчитайте массу навески анализируемого вещества и объем осадителя.
Вариант 20
1. Весовойанализ.Основныеоперацииданногометода.Какосуществляютопределениекристаллизационнойводывкристаллогидрате?Опишитеходданногоопределения.
2. Вычислитепроцентноесодержаниекристаллизационнойводывмедномкупоросепоследующимданным:массапустоготигля3,52456г,массатигляснавеской4,74115г,массатигляснавескойпослевысушивания4,30355г.
Вариант 21
1. Дайте определение понятиям: инклюзия и окклюзия.
2. Какойобъем0,5нрастворасернойкислотыпотребуетсядляосажденияионовбарияизнавескинитратабариявеличиной0,5г?
Вариант 22
1. Для чего применяют переосаждение?
2. Дляанализавзято0,8500гхлоридабария.Приегообработкесернойкислотойобразовалсяосадоксульфатабариявколичестве0,5640г.Сколькограммовбариявходитвсоставосадка?Определитьпроцентноесодержаниебариявовзятойнавеске.
Вариант 23
1. Что такое пептизация в методе осаждения?
2. Нааналитическихвесахотвесилинавескув0,1680гх.ч.хлоридакальция.Приготовилиизнее150млраствора.Определитеэквивалентнуюконцентрациюититрданногораствора.
Вариант 24
1. Требования, предъявляемые к осажденной и гравиметрической форме.
2. Дляопределениясодержаниясульфатакалиягравиметрическимметодомиз5,5гобразца,содержащегосульфаткалия,былополучено2,12гсульфатабария.Определитемассовуюдолюсульфатакалиявобразце.
Вариант 25
1. Какие экспериментальные измерения необходимы при расчете в гравиметрии?
2. ОпределитесодержаниебариявобразцехимическичистогоBaCl2∙2H2O,еслинавескачистогоBaCl2∙2H2Oравна0,6880г.Массаосадкасульфатабарияпослепрокаливаниясоставила0,6655г.
Вариант 26
1. Как вычислить массовую процентную долю w,% определяемого компонента в навеске?
2. Прианализе0,5105гсплаваполучено0,1008гAl2O3.Определитепроцентноесодержаниеалюминиявсплаве.
Вариант 27
1. Что такое гравиметрический фактор и как его можно рассчитать?
2. Приопределениижелезавесовымметодомиз5,5гвеществабылополучено0,58гFe2O3.Чемуравнамассоваядоляжелезавобразце?
Вариант 28
1. Посуда и оборудование, применяемые в гравиметрическом анализе?
2. Сколькомл0,1нраствораоксалатааммонияпотребуетсядляосажденияионакальцияизраствора,полученногоприрастворении0,5гкарбонатакальция?
Вариант 29
1. Перечислитеосновныетребованиякосадку.Отличиеосаждаемойформыотвесовой.
2. Дляопределениясодержаниясульфатакалиягравиметрическимметодомиз13,5гобразца,содержащегосульфаткалия,былополучено3,12гсульфатабария.Определитемассовуюдолюсульфатакалиявобразце.
Вариант 30






