ѕример. ¬озможна ли следующа€ реакци€ в стандартных услови€х:
t
Siќ2 (к) + 2NaќH (p) = Na2Siќ3 (к) + Ќ2ќ (ж)
если Δ G о(Siќ2 (к)) = Ц803,75 кƒж/моль; Δ G о (Na2Siќ3 (к))= Ц1427,8 кƒж/моль;
Δ G о (NaќH(p)) = Ц419,5 кƒж/моль; Δ G о (Ќ2ќ (ж)) = Ц237,5 кƒж/моль?
ћожно ли выпаривать щелочь в стекл€нном сосуде?
–ешение. »зменение энергии √иббса Δ G о298 реакции равно:
Δ G о = Σ G опрод. Ц Σ G оисх.;
Δ G о298 = (Ц1427,8 Ц 237,5) Ц (Ц803,75 Ц419,5Ј2)= Ц22,55 кƒж;
Δ G о298 = Ц22,55 кƒж (т. е. Δ G <0), а следовательно, данна€ реакци€ возможна. ўелочь нельз€ выпаривать в стекл€нном сосуде, так как в состав стекла входит Siќ2.
ѕример. ¬ычислить Δ G о дл€ реакции 2Ќ2 (г)+ќ2 (г)  2Ќ2ќ(г).
2Ќ2ќ(г).
при 298, 500, 1000, 1500 . «ависимостью Δ Ќ о и Δ S o от температуры пренебречь. ѕостроить график зависимости Δ G о от температуры и найти по графику температуру, ниже которой указанна€ реакци€ в стандартных услови€х может протекать самопроизвольно.
–ешение. —огласно уравнению Δ G = Δ Ќ Ц T Δ S вли€ние температуры на Δ G определ€етс€ знаком и величиной Δ S. ≈сли пренебречь вли€нием “ на значени€ Δ Ќ и Δ S, то приведЄнна€ зависимость Δ G = ƒ(T) €вл€етс€ уравнением пр€мой, наклон которой определ€етс€ знаком Δ S. при Δ S >0 пр€ма€ идет вниз, при Δ S <0 Ц вверх.
ќпределим величину ΔЌ∞298 (исходные данные берем из табл.1)
∆ H ºp = Σ∆ H ºобр.(прод.) Ц Σ∆ H ºобр.(исх..)
ΔЌ∞298 = 2ΔЌ∞обр.(H2O) Ц (2ΔЌ∞обр.(H2) + 2ΔЌ∞обр.(O2) = 2ΔЌ∞обр.(H2O) =
=2(-241,84) = Ц483,68 (кƒж) (на 2 мол€ H2O)
ΔЌ∞обр.(Ќ2ќ) = 0,5(Ц483,8) = Ц241,89 кƒж/моль.<0
—ледовательно, реакци€ экзотермическа€.
ќпределим изменение энтропии данной реакции в стандартных услови€х ΔS∞298 (исходные данные берем из табл.1)
Δ S о = Σ S опрод. Ц Σ S оисх.:
ΔS∞298= 2S∞298.(H2O) Ц [2S∞298.(H2) + S∞ 298.(O2)]= 2Ј188,74 Ц (2Ј130.6 + 205) =
Ц98,6(ƒж/ ) = Ц0,0986(кƒж/ ) < 0, Δ G = ƒ(T) пр€ма€ идет вверх.
ќпределим изменение энергии √иббса ΔG∞298 в стандартных услови€х (исходные данные берем из табл.1):
Δ G о = Σ G опрод. Ц Σ G оисх.;
ΔG∞298 = 2ΔG∞298.(H2O) Ц [2ΔG∞298(H2) Ц ΔG∞298(O2)] = 2(Ц228,8) = Ц457,6 кƒж.
ќтрицательна€ величина ΔG∞298 свидетельствует о том, что в стандартных услови€х реакци€ самопроизвольно протекает в пр€мом направлении.
ΔG∞298 = ΔЌ∞298 Ц 298ЈΔS0298 = Ц483,68 Ц 298Ј(Ц0,0986) = Ц457,6кƒж
ΔG∞500 = ΔЌ∞298 Ц 500ЈΔS0298 = Ц483,68 Ц 500Ј(Ц0,0986) = Ц434,38кƒж
ΔG∞1000 = ΔЌ∞298 Ц 1000ЈΔS0298 = Ц483,68 Ц 1000Ј(Ц0,0986) = Ц385,08кƒж
ΔG∞1500 = ΔЌ∞298 Ц 1500ЈΔS0298 = Ц483,68 Ц I500Ј(Ц0,0986) = Ц335,78 кƒж
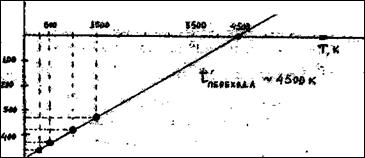
ѕостроим график ΔG∞“ =f(“):
|
|
|
 ΔG∞“
ΔG∞“
“емпература перехода ~4500
–≈ ќћ≈Ќƒј÷»» ƒЋя —јћќ—“ќя“≈Ћ№Ќќ… –јЅќ“џ —“”ƒ≈Ќ“ќ¬ » ¬ј–»јЌ“џ «јƒјЌ»…
–≈ ќћ≈Ќƒј÷»»:
1. ¬нимательно изучить теоретический материал, использу€ конспекты лекций, данное учебное пособие и рекомендуемую литературу.
2. ѕроверить усвоение теории, ответив на контрольные вопросы, выполнив тестовые задани€.
3. –азобрать примеры решени€ типовых задач.
«јƒј„» ƒЋя —јћќ—“ќя“≈Ћ№Ќќ√ќ –≈Ў≈Ќ»я
«адачи
1. ¬ычислите стандартную теплоту образовани€ бензола —6Ќ6 (ж), если известны теплоты сгорани€ водорода, углерода и бензола.
2. ќпределите стандартную теплоту образовани€ сероуглерода CS2, если известно, что
CS2 (ж) + 3ќ2 = —ќ2 (г) + 2Sќ2 (г) Δ Ќ о298 = Ц1075 кƒж/моль.
3. ¬ычислите Δ Ќ о298 хлорида аммони€, если дл€ реакции
NH3 (г) + Ќ—1(г) = NH4CI (к)
Δ Ќ о298 = Ц176,93 кƒж/моль.
4. ќпределите Δ Ќ о298 BiCl3(к), если Δ Ќ о298BiCl3(г) = Ц270,70 кƒж/моль, а Δ Ќ о возгонки BiCl3(к) 113,39 кƒж/моль.
5. ѕри взаимодействии 5 г металлического натри€ с водой выдел€етс€ 40,25 кƒж теплоты, а при взаимодействии 10 г оксида натри€ с водой выдел€етс€
36,46 кƒж теплоты. –ассчитайте Δ Ќ о298 Na20.
6. ѕри растворении 16 г —а—2 в воде выдел€етс€ 31,3 кƒж теплоты. ќпределите стандартную теплоту образовани€ —а(ќЌ)2.
7. ќпределите Δ Ќ о298 Fe2ќ3, если при реакции
2Fe + ј12ќ3 = Fe2ќ3+ 2A1
на каждые 80 г Fe2ќ3 поглощаетс€ 426,5 кƒж теплоты.
8. “епловой эффект реакции
Sќ2(г) + 2H2S(г) = 3S(ромб) + 2Ќ2ќ(ж)
равен Ц234,50 кƒж. ќпределите стандартную теплоту образовани€ H2S.
9. ќкисление аммиака протекает по уравнению
4NH3 (г) + 3ќ2 (г) = 2N2 + 6Ќ20(ж) Δ Ќ о298 = Ц1528 кƒж.
ќпределите стандартную теплоту образовани€ NH3(г) и NH4ќH, если теплота растворени€ NH3(г) в воде равна Ц34,65 кƒж.
10. ¬ычислите стандартную теплоту образовани€ сахарозы —12Ќ22ќ11, если тепловой эффект реакции
—12Ќ22ќ11 + 12ќ2 = 12—ќ2 + 11Ќ2ќ(ж)
равен Ц5694 кƒж.
11. –ассчитайте Δ Ќ о298 ZnSќ4, если известно, что
2ZnS + 3ќ2 = 2Znќ + 2Sќ2 Δ Ќ о = Ц890,0 кƒж;
2Sќ2 + ќ2 = 2Sќ3 Δ Ќ о = Ц196,6 кƒж;
ZnSќ4 = ZnO + Sќ4 Δ Ќ о = +234,0 кƒж.
12. ¬осстановление диоксида свинца водородом протекает по уравнению
–bќ2 + Ќ2 = –bќ + Ќ2ќ(г) Δ Ќ о = Ц182,8 кƒж.
ќпределите стандартную теплоту образовани€ –bќ2.
13. ¬ычислите стандартную теплоту образовани€ бензойной кислоты —6Ќ5—ќќЌ(к), если стандартна€ теплота сгорани€ бензойной кислоты равна
Ц3227,54 кƒж/моль, а стандартные теплоты образовани€ Ќ2ќ и —ќ2 см. в табл. 1 приложени€.
14. ¬ычислите теплоту образовани€ карбида кальци€ CaC2, исход€ из теплового эффекта реакции
Caќ + 3— = CaC2 + Cќ; Δ Ќ о = 460,0 кƒж.
15.ќпределите Δ Ќ о298 образовани€ этилена, использу€ следующие данные:
C2H4 (г) + 3O2 (г) = 2CO2 (г) + 2H2O(г), Δ Ќ о = Ц1323 кƒж;
C(графит) + O2 (г) = CO2 (г) Δ Ќ о = Ц393,5 кƒж;
H2(г) + ½ O2(г)=H2O(г); Δ Ќ о = Ц241,8 кƒж
16. ¬ычислите Δ Ќ о реакций. ”кажите, кака€ из них €вл€етс€ эндотермической, а кака€ экзотермической. “епловой эффект каких реакций представл€ет собой теплоту сгорани€ вещества?
| а) 4NH3 (г)+5O2 (г)→4NO(г)+6H2O(г); | к) 2H2S(г)+SO2(г)→3S(ромб)+2H2O(ж); |
| б) 4NH3 (г)+3O2 (г)→2N2 (г)+6H2O(г); | л) 2H2(г)+P4(т)→4PH3(г); |
| в) Fe2O3 (к)+3CO(г)→2Fe(к)+3CO2 (г); | м) 3Fe(т)+2O2(г)→Fe2O3(к); |
| г) CH4 (г)+2O2 (г)→CO2 (г)+H2O(ж); | н) 2C2H2 (г)+5ќ2 (г)→4CO2 (г)+2H2O(г); |
| д) 2Mg(к)+CO2 (г)→2MgO(к)+C(графит); | п) 4HF(г)+Br2 (ж)→2HBr(ж)+F2 (г); |
| е) 2—l2 (г)+H2O(г)→4HCl(г)+O2 (г); | р) CaO(к)+CO2 (г)→CaCO3 (к); |
| ж) 3CH4 (г)+CO2 (г)+H2O(ж)→4CO(г)+8H2 (г); | с) 4—ќ(г) + 2SO2 (г)→4—ќ2 (г) + S2 (г). |
| и) CaO(к)+SiO2(к)→CaSiO3 (к); |
ак необходимо было бы записать уравнени€ некоторых реакций (каких?), чтобы теплота этих реакций могла бы быть названа теплотой сгорани€?
|
|
|
17. ¬ычислите тепловой эффект реакции
јI2O3 (к) + 3SO3 (г) = Al2(S04)3 (к),
если известны стандартные теплоты образовани€ реагирующих веществ.
18. «на€ стандартные теплоты сгорани€ этана, метана и водорода (см. табл. 2 приложени€), определите Δ Ќ о реакции:
—2Ќ6 (г) + Ќ2 (г) = 2—Ќ4 (г)
19. »спользу€ значение Δ Ќ о298 реагирующих веществ, определите тепловой эффект реакции восстановлени€ оксидом углерода оксида свинца (IV) до оксида свинца (II) с образованием диоксида углерода.
20. ѕо стандартным теплотам сгорани€ веществ рассчитайте Δ Ќ о298 системы
—2Ќ5ќЌ (ж) + —H3—ќќЌ (ж) = —Ќ3—ќќ—2Ќ5 (ж) + Ќ2O
Δ Ќ осгор..сн3соос2н5 = Ц2254,21 кƒж/моль.
онечные продукты сгорани€ Ц газообразный —ќ2 и жидка€ Ќ2ќ.
21. ќпределите тепловой эффект реакции
NaH (к) + Ќ2ќ (ж) = NaOH (p) + Ќ2 (г)
по стандартным теплотам образовани€ веществ, участвующих в реакции, если Δ Ќ о NaH (к) = Ц56,94 кƒж/моль, Δ Ќ о NaќЌ (р) = Ц469,47 кƒж/моль.
22. ќпределите тепловой эффект реакции
2PbS +3ќ2 = 2–bO + 2Sќ2 ,
использу€ значение стандартных теплот образовани€ реагирующих, веществ.
23 ¬ычислите теплоту перехода графита в алмаз, если при образовании
одного мол€ —ќ2 из графита выдел€етс€ 393,5 кƒж/моль, а из алмаза Ц 395,4 кƒж/моль.
24. »сход€ из реакций
—Iќ3 = —1 + ½ќ2; Δ Ќ о = Ц49,4 кƒж/моль,
KCIќ4 = —1 + 2ќ2; Δ Ќ о = 33 кƒж/моль,
вычислите Δ Ќ о реакции
4K—Iќ3 = 3KCIќ4 + —1
25. “еплоты сгорани€ этана —2Ќ6 и этилена —2Ќ4 соответственно составл€ют Ц1560 и
Ц1411 кƒж/моль. ¬ычислите Δ Ќ о298 реакции гидрировани€ этилена
—2Ќ4 + Ќ2 = —2Ќ6
26. “еплоты сгорани€ бензола (г) и ацетилена соответственно составл€ют Ц3268 и
Ц1301 кƒж/моль. ¬ычислите Δ Ќ о298 реакции 3—2Ќ2 (г) = —6Ќ6 (г)
27. “еплота сгорани€ этилового спирта составл€ет Ц1409 кƒж/моль. ¬ычислите Δ Ќ о298 реакции
2—ќ + 4Ќ2 = —2Ќ5ќЌ + Ќ2ќ(ж)
28. ¬ычислите Δ Ќ о298 реакции:
а) 2Li (к) + 2H2O (ж) = 2Li+(водн.) + 2ќЌЦ(водн.) + Ќ2 (г);
б) 2Nа (к) + 2H2O (ж) = 2Nа+(водн.) + 2ќЌЦ(водн.) + Ќ2 (г);
—тандартные энтальпии образовани€ Li+(водн.), Nа+(водн.), ќЌЦ(водн.) прин€ть соответственно равными Ц278,5, Ц 239,7 и Ц228,9 кƒж/моль.
29. »сход€ из Δ Ќ о298 образовани€ H2O (г) и следующих данных:
FeO (к) + CO (г) = Fe(к) + —ќ2 (г) Δ Ќ о298 = Ц18,2 кƒж;
2CO (г) + ќ2 = 2—ќ2 (г) Δ Ќ о298 = Ц566,0 кƒж;
вычислить Δ Ќ о298 реакции
FeO (к) + H2 (г) = Fe (к) + H2O (г).
30. ќпределить Δ Ќ о298 реакции 3—2Ќ2 (г) = —6Ќ6 (ж), если Δ Ќ о298 реакции горени€ ацетилена с образованием —ќ2 (г) и Ќ2O (ж) равна Ц1301 кƒж/моль, а Δ Ќ о298 образовани€ —6Ќ6 (ж) составл€ет 82,9 кƒж/моль.
31. ѕри стандартных услови€х теплота полного сгорани€ белого фосфора равна
760,1 к ƒж/моль, а теплота сгорани€ черного фосфора равна 722,1 к ƒж/моль. „ему равна теплота превращени€ черного фосфора в белый при стандартных услови€х?
32. ѕри получении азотной кислоты из KNќ3 протекают следующие реакции:
KNќ3 (к) + H2Sќ4 (р) = KHSќ4 (к) + HNќ3 (г) (а)
2KNќ3 (к) + H2Sќ4 (р) = K2Sќ4 (к) + 2HNќ3 (г) (б)
—колько теплоты выдел€етс€ (или поглощаетс€) при получении 1 кг азотной кислоты, если 80% ее образуетс€ по реакции (а), Δ Ќ о (HNќ3(г))=
Ц133,90 кƒж/моль.
33. –азложение гремучей ртути при взрыве идет по уравнению
|
|
|
Hg(ONC)2 = Hg + 2CO + N2 + 364,2 кƒж.
ќпределите объем выделившихс€ газов (н.у.) и количество теплоты, поглотившейс€ при взрыве 1,5 кг Hg(ONC)2.
34. ќпределите количество теплоты, выдел€ющейс€ при взаимодействии 50 г фосфорного ангидрида с водой по реакции
–2O5 + Ќ2ќ = 2Ќ–O3,
если тепловые эффекты реакции равны:
2– + 5/2O2 = –2O5 Ц1549,0 кƒж;
2– + Ќ2 + 3O2 = 2Ќ–O3 Ц1964,8 кƒж.
35. ¬ычислите количество теплоты, которое выдел€етс€ при сгорании 20 л диборана (н.у.), если Δ Ќ о298¬203 (к) и ¬2Ќ6 (г) соответственно равны Ц1264 и +31,4 кƒж/моль. ÷елесообразно ли использовать в качестве топлива диборан вместо этана, если стандартна€ теплота сгорани€ этана Ц1559,88 кƒж/моль?
36. Ќайдите теплоту сгорани€ алмаза, если стандартна€ теплота сгорани€ графита равна Ц393,51 кƒж/моль, а теплота фазового перехода
— (графит) → — (алмаз) равна 1,88 кƒж/моль.
37. акое количество теплоты выдел€етс€ при превращении 1 кг красного фосфора в черный, если Δ Ќ о– (красный) = Ц18,41; Δ Ќ о– (чЄрный)= Ц43,20 кƒж/моль?
38. —колько нужно затратить теплоты, чтобы разложить 200 г Na2Cќ3 до оксида натри€ и диоксида углерода, если тепловые эффекты реакций равны:
Na2Cќ3 + Siќ2 = Na2Siќ3 + —ќ2 +819,29 кƒж;
Na2ќ + Siќ2 = Na2Siќ3 Ц243,5 кƒж?
39. —колько теплоты выделитс€ при сжигании 38 г сероуглерода CS2?
40. ѕри полном сгорании этилена (с образованием жидкой воды) выделилось 6226 кƒж. Ќайти объем вступившего в реакцию кислорода (услови€ нормальные).
41. ¬од€ной газ представл€ет собой смесь равных объемов водорода и оксида углерода (II). Ќайти количество теплоты, выдел€ющейс€ при сжигании 112 л вод€ного газа, вз€того при нормальных услови€х.
42. —ожжены с образованием H2O (г) равные объемы водорода и ацетилена, вз€тых при одинаковых услови€х. ¬ каком случае выделитс€ больше теплоты? ¬о сколько раз?
43. Ќайти массу метана, при полном сгорании которой (с образованием жидкой воды) выдел€етс€ теплота Q, достаточна€ дл€ нагревани€ 100г воды от 20 до 30º—. ћольную теплоемкость воды прин€ть равной — = 75,3 ƒж/(мольЈ ).
Q = CЈνЈΔt, где ν Ц число молей воды.
44. Ќайти количество теплоты, выдел€ющеес€ при взрыве 8,4 л гремучего газа (2Ќ2 + ќ2), вз€того при нормальных услови€х.






