СБОРНИК ЗАДАНИЙ
ДЛЯ САМОСТОЯТЕЛЬНОЙ РАБОТЫ СТУДЕНТОВ ПО ДИСЦИПЛИНЕ «ХИМИЯ»
Федеральное агентство по образованию
Государственное образовательное учреждение высшего
Профессионального образования
«Уфимский государственный нефтяной технический университет»
СБОРНИК ЗАДАНИЙ
ДЛЯ САМОСТОЯТЕЛЬНОЙ РАБОТЫ СТУДЕНТОВ ПО ДИСЦИПЛИНЕ «ХИМИЯ»
Учебно-методическое пособие
Под общей редакцией доцента Саловой Л.Е.
Уфа 2008
УДК 54(07)
ББК 24.1 я7
С 23
Утверждено Редакционно-издательским советом УГНТУ в качестве учебно-методического пособия
Авторы: Л.Е.Салова, Ю.И.Пузин, О.Б.Чалова, М.Н.Назаров, С.С.Злотский, Л.Н.Зорина, А.Т.Чанышева, Ф.Н.Латыпова, Л.З.Рольник, М.А.Молявко, Л.Г.Сергеева, О.И.Михайленко, С.Б.Денисова, О.Ф.Булатова, Ф.Б.Шевляков
Р ецензент. канд. хим. наук проф. А.М.Сыркин
Сборник заданий для СРС по дисциплине «Химия»: Учебно-методическое пособие. - Уфа: Изд-во УГНТУ, 2008.-с.
ISBN 5-7831-0780-8
Настоящее учебно-методическое пособие является сборником заданий для самостоятельной работы студентов первого курса нехимических специальностей. Оно включает задания в в идее вопросов и задач, примеры решений, варианты заданий и списки рекомендуемой литературы к каждой теме сборника
УДК 54(07)
ББК 24.1 я7
ISBN 5-7831-0780-8 © Уфимский государственный нефтяной
технический университет, 2008
© Салова Л.Е., Ю.И.Пузин, С.С.Злотский, Л.З.Рольник, О.Б.Чалова, М.Н.Назаров, Л.Н.Зорина, А.Т.Чанышева, Ф.Н.Латыпо-ва, М.А.Молявко, Л.Г.Сергеева, О.И.Ми-хайленко, С.Б.Денисова, О.Ф.Булатова, Ф.Б.Шевляков,
Содержание
| С. | ||
| Задание 1. Строение вещества | ||
| Задание 2. Термохимия. Направление химических реак-ций | ||
| Задание 3. Химическая кинетика и равновесие | ||
| Задание 4. Растворы | ||
| Задание 5. Растворы электролитов | ||
| Задание 6. Гидролиз солей | ||
| Задание 7. Окислительно-восстановительные реакции. Электрохимия | ||
| Задание 8. Классификация неорганических веществ. Свойства | ||
| Задание 9. Химия воды. Жесткость воды | ||
| Задание 10. Дисперсные системы |
ЗАДАНИЕ №1
по теме: "СТРОЕНИЕ ВЕЩЕСТВА",
Таблица 1. Варианты домашнего задания по теме «Строение вещества
| . Номер вари-анта | Задание 1 | Задание 2 | Задание 3 | ||
| поряд-ковый номер эле-мента | номер электрона в атоме | Задание 3.1 (Номер задачи) | Задание 3.2 | ||
| I | (1, 3, 10, 15, 20) | CF4; SiF62- | Ar; Cu | ||
| (4, 7, 21, 29, 30) | NF3; ICl4+ | CCl4; CaCl2 | |||
| (2, 6, 12 31, 40) | H2O2; PCI6- | S8; Mg | |||
| (5, 11, 18, 37, 47) | SO3; PO43- | P4; CuCl2 | |||
| (9, 13, 19, 21, 22) | PF3Cl2,H3PO3 | C2H2Cl2; W | |||
| (10, 16, 27, 31, 32) | HNO2; CH3OCH3 | NH3; Li3N | |||
| (14, 21, 28, 33, 39) | CH3CH2OH; SO2 | FeCl3; Fe | |||
| (5, 10, 15, 20,26) | CHCl3; SO42- | CH3OH; SiO2 | |||
| (4, 8, 12, 17,22) | HClO3; CO32- | CH2O; MgO | |||
| (17, 21, 23,29, 32) | ClO2-; SCl4 | C2H5OH; K2SO4 | |||
| (4, 14, 24, 34, 43) | H2Se; COCl2 | CH3COOH; SiC | |||
| (5, 11,17, 23, 51) | AsH3; NO2 | CH2Cl2; Ni | |||
| (8,12, 20, 21, 24) | SiCl4; H2SO3 | COF2; MgF2 | |||
| (3, 7, 13, 20, 32) | SCl2; HNO2 | Ti; TiCl2 | |||
| (7, 14, 19, 25, 43) | SOCl2; HNO3 | HF; KF | |||
| (8. 18, 28, 38. 48) | KClO3; SO32-; | BF3; BN | |||
| (7, 12, 16, 20, 25) | H2SO4; C2H3F | CH3CHO; Cr | |||
| (3, 11, 19, 31, 34) | SO2; HNO3 | COCl2; KCl | |||
| (6, 16, 26, 36, 45) | Na2SO3; HClO4 | HCOOH; Ne | |||
| (21, 23, 27, 37, 48) | CH3СOOН; IBr3 | Ni; NiCl2 | |||
| (7, 9, 21, 23, 26) | CH3CH2СOOН; SiF62-; | C2H4Cl2; V | |||
| (31, 33, 35, 37, 38) | C2H4Cl2; BrF5 | Br2; KBr | |||
| (14, 24, 34, 44, 46) | C2H2 ; SF6 | CHCl3; Zn | |||
| (12, 23, 35, 48, 54) | H2CO3; CH3СOONa | CH3NH2; Ag | |||
| (3, 13, 17, 21, 26) | HCCl3 ; HClO4 | SiF4; Na2S |
Задание 1 по теме: «СТРОЕНИЕ АТОМА»
1.1 Напишите электронно-графическую формулу атома элемента с указанным порядковым номером. Впишите в таблицу значения квантовых чисел, характеризующих электроны в основном состоянии: (в задании указаны номера электронов в порядке заполнения атомных орбиталей)
| Номер электрона | Значение квантовых чисел | ||
| n | l | ml | ms |
Какие из четырех квантовых чисел определяют энергию электрона в атоме? Какие из них характеризуют форму орбитали и её расположение в пространстве?
1.2. Укажите тип элемента (s-, p-, d-, f-), он относится к металлам или неметаллам. Укажите валентные электроны атома данного элемента. Каковы его валентные возможности? Если возбужденные состояния возможны для данного атома, то запишите их с помощью электронных формул. Если невозможны - объясните почему. Сколько неспаренных электронов имеется в атоме в основном состоянии и сколько – в возбужденном состояниях? Сколько вакантных орбиталей имеется в атоме в основном и возбужденном состояниях? Определите высшую и низшую степени окисления атома данного элемента. Какие свойства - окислительные, восстановительные или и те и другие - будет проявлять атом в высшей, низшей и других степенях окисления?
1.3. Какие ионы может образовать атом данного элемента? Запишите их электронные формулы. Как изменяются их ионные радиусы? Приведите примеры изоэлектронных частиц.
1.4. Проанализируйте характер изменения первых пяти энергий (потенциалов) ионизации на основании изменения заряда частицы и её радиуса. Запишите схемы происходящих процессов. Подтвердите, по возможности, выводы справочными значениями энергий ионизации.
1.5. Определите электронные аналоги элемента и составьте их электронные формулы. Запишите общую электронную формулу валентных электронов для элементов данной подгруппы. Чем объяснить сходство в химических свойствах этих элементов? Как изменяются свойства атомов элементов одной подгруппы (радиусы, энергия ионизации, энергия сродства к электрону, электроотрицательность, окислительно-восстановительные свойства)? Подтвердите свои выводы справочными данными.
1.6. Как изменяются свойства (см.п.1.5) атомов данного элемента по сравнению с элементами-соседями по периоду? Свои выводы, по возможности, подтвердите справочными данными.
Методические указания к выполнению задания 1.
Вам выдано домашнее задание в виде нескольких чисел, например, 20 (2,4,6,18,19). Первая цифра означает № элемента в Периодической системе. Следовательно, элемент №20 -кальций, химический символ "Са". Цифры в скобках указывают номера электронов в оболочке атома калъция, состояние которых надо охарактеризовать с помощью четырех квантовых чисел. Теперь переходим к выполнению задания 1 по пунктам 1 - 6.
1.1. Запишем электронно-графическую формулу атома элемента №20, найдем и подчеркнем указанные в задании пять электронов в его оболочке (пользуясь правилом Гунда):
а) полная электронная формула:
20Ca 1s2 2s2 2p63s23p64s2
б) краткая электронная формула:
20Ca [Ar]4s2
в) электронно-графическая формула
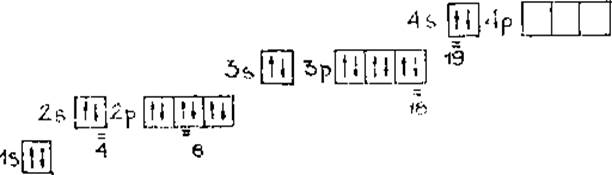

 2
2
Определим квантовые числа для заданных электронов в атоме кальция и составим таблицу 2.
Таблица 2 Значения квантовых чисел для электронов в атоме кальция.
| Номер электрона | Значения квантовых чисел | |||
| n | l | ml | ms | |
| -1/2 | ||||
| -1/2 | ||||
| +1/2 | ||||
| +1 | -1/2 | |||
| +1/2 |
При определении ml исходим из предположения, что орбитали каждого подуровня заполняются в порядке увеличения ml от наименьшего отрицательного значения к наибольшему положительному т.е. при l=1 ml изменяется [–1,0.1], следовательно, для p-подуровня
 |
px py Pz
для d-подуровня
|
|
При определении ms условно принимаем направление стрелочки вверх за положительное значение спинового числа, то есть ms = + ½; вниз - за отрицательное, то есть ms = - l/2.
1.2. Существует четыре типа элементов s-, p-,d- f-. Валентные электроны – это электроны внешнего электронного слоя, а также предвнешнего подуровня, который заполняется в данном атоме. Таким образом, валентные электроны Са – 4s2, и следовательно, Са - s- элемент, металл. В основном состоянии кальций о-валентен, так как не имеет неспаренных электронов. Возбуждение возможно, так как на внешнем уровне есть вакантные орбитали: 20Са* 1s22s22p63s23p64s14p1.



Ca*…4s 4р
В возбужденном состоянии атом кальция содержит два неспаренных электрона, поэтому в соединениях кальций двухвалентен.
Таблица 3. Валентные возможности атома кальция
| Показатель | Основное состояние | Возбужденное состояние |
| Число неспаренных | нет | |
| Число вакантных орбиталей | 15(4px4py4pz;4d; 4f) | 14 (4py 4pz; 4d; 4f) |
| Высшая степень окисления | - | +2 |
| Низшая степень окисления | - |
1.3. Характерные степени окисления элементов, его валентности и наиболее устойчивые ионы, которые он может образовать, определяются конфигурацией валентных электронных слоев.. Атом кальция может образовать только ион Са2+, так как на внешнем слое у него только 2 валентных электрона. Его электронная формула 1s22s22р63s23р6; ионный радиус составляет 0.97А.
Изоэлектронными являются частицы, имеющие одинаковую электронную конфигурацию. Поэтому изоэлектронными по отношению к иону Са2+ будут следующие частицы:
| К+ | 1s22s22p63s23p6 | |
| Аг | ls22s22p63s23p6 | |
| Cl- | 1s22s22p63s23p6 | |
| S2- | ls22s22p63s23p6 | |
| Sс3+ | 1s22s22p63s23p6 | |
| Ti4+ | 1s22s22p63s23p6 |
При ответе на этот вопрос для d– и f- элементов, полезно просмотреть учебную литературу по химии элементов.
1.4. Запишем схемы процессов последовательного отрыва пяти электронов от атома кальция, которым соответствуют первые пять энергий (потенциалов) ионизации:
Са° - е = Са+ I1 = 6.11 эВ
Са+ - е = Са2+ I2 = 11.87 эВ
Са2+ - е = Са3+ I3 = 51.0 эВ
Са3+ - е = Са4+ I4 = нет данных
Са4+ - е = Са5+ I5 = нет данных
С отрывом каждого последующего электрона увеличивается заряд частицы от 0 до +5 и уменьшается её радиус, так как при одном и том же заряде ядра число электронов убывает, и оставшиеся сильнее притягиваются к ядру. Поэтому каждый последующий потенциал больше, чем предыдущий. Резкое увеличение потенциала ионизации происходит при отрыве электрона с внутреннего уровня, например, сравним I3 = 51,0эВ >> I2 = 11,87эВ
1.5. Электронными аналогами являются элементы, имеющие подобные конфигурации валентных электронных слоев. Они могут быть описаны общей электронной формулой, и являются элементами одной подгруппы Периодической системы.
Электронные аналоги кальция: Be, Mg, Sr, Ba, Ra. Общая электронная формула валентных электронов: Э...ns2. Все элементы - металлы, относятся ко 2 группе, главной подгруппе. Радиусы атомов элементов с увеличением заряда ядра в подгруппе (в направлении сверху вниз) увеличиваются, энергия ионизации, сродство к электрону, электроотрицателъность в этом направлении уменьшается, восстановительная способность увеличивается (таблица 4).
Таблица 4. Изменение свойств атомов элементов одной подгруппы.
| Элемент | Атомные радиусы, rат. Ао | Энергия ионизации I1, эВ | Энергия сродства к е Е, эВ | Электроотрицательность (по Полин-гу) |
| Be | 1,13 | 9,32 | 0,19 | 1.5 |
| Mg | 1,60 | 7,64 | -0,32 | 1.2 |
| Са | 1,95 | 6,11 | - | 1,0 |
| Sr | 2,15 | 5,69 | - | 1,0 |
| Ва | 2.21 | 5,21 | - | 0,9 |
| Rа | 2,35 | 5,28 | - | - |
1.6. Элементами-соседями кальция по периоду являются К и Sс. Их свойства приведены в таблице 5.
Таблица 5. Изменение свойств атомов элементов одного периода.
| Свойства | К (К+) | Ca(Ca2+) | Sc(Sс3+) |
| Атомные радиусы, А0 | 2,31 | 1,97 | 1,6 |
| Ионные радиусы, А0 | 1,33 | 0,97 | 0,81 |
| Энергия ионизации I1, эВ | 4,34 | 6,11 | 6,54 |
| Энергия сродства к е-, эВ | 0,82 | - | - |
| Электроотрицательность (по Полингу) | 0,80 | 1,3 |
увеличиваются окислительные свойства ионов

 увеличиваются восстановительные свойства металлов
увеличиваются восстановительные свойства металлов
Задание 2 по теме:






