Принцип действия кондуктометров основан на измерении электропроводности анализируемого вещества, являющейся функцией концентрации какого-либо компонента. Основным элементом контактных кондуктометров является электродная ячейка (рисунок 3.1), включаемая в одно из плеч неуравновешенного моста.
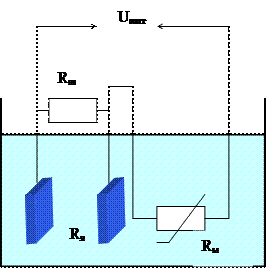
Рисунок 3.1 – Электродная ячейка кондуктометра
Каждая ячейка имеет свою постоянную К, т.е. коэффициент, определяющий соотношение между удельной электропроводностью χ0 анализируемого раствора и электропроводностью χ ячейки или ее сопротивлением Rя, под которым понимается сопротивление жидкости, заполняющей межэлектродное пространство:
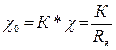 . (3.1)
. (3.1)
Размерность постоянной ячейки [K] = м-1.
Электропроводность раствора очень сильно зависит от температуры, поэтому последовательно с электродами включается медный терморезистор Rм. Для температурной компенсации необходимо, чтобы изменение сопротивления электродной ячейки ΔRя было равно по величине и противоположно по знаку изменению сопротивления медного терморезистора ΔRм. Для узкого интервала температур зависимость сопротивления ячейки от температуры имеет вид
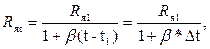 (3.2)
(3.2)
где t – текущее значение температуры в интервале t1 – t2; β – средний температурный коэффициент электропроводности раствора в этом интервале температур; Rяt и Rя1 – сопротивления ячейки при значениях температуры t и t1.
Изменение сопротивления ΔRя ячейки при изменении температуры от t1 до t2 равно
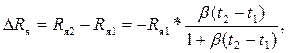 (3.3)
(3.3)
т.е. сопротивление нелинейно уменьшается с ростом температуры.
Изменение сопротивления ΔRм медного резистора при изменении его температуры от t1 до t2 равно
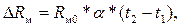 (3.4)
(3.4)
где Rм0 – сопротивление резистора при 0 0С; α = 0,00426 1/0С – температурный коэффициент сопротивления меди.
Таким образом, из-за нелинейного изменения ΔRя и линейного изменения ΔRм полная компенсация возможна не более чем в двух точках температурного диапазона t1 - t2.
При небольших концентрациях растворов их электропроводность χ мала, поэтому для уменьшения расчетного значения Rм0 электроды ячейки шунтируются манганиновым сопротивлением Rш. Их эквивалентное сопротивление называется сопротивлением измерительной ячейки, или приведенным сопротивлением электродной ячейки Rпр. Оно будет равно
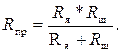 (3.5)
(3.5)
При изменении температуры раствора от первоначального значения t1 до текущего t приведенное сопротивление Rпрt станет равным
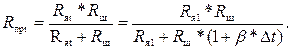 (3.6)
(3.6)
Эта зависимость также нелинейная, поэтому полная температурная компенсация по-прежнему возможна только в двух точках.
Условием компенсации является равенство ΔRпр и ΔRм. Для Δt = t1 – t2 изменение ΔRпр равно
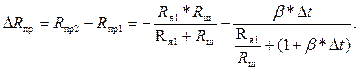 (3.7)
(3.7)
Значение температурной погрешности неодинаково в рабочем интервале температур. Максимальная погрешность будет иметь место, когда
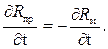 (3.8)
(3.8)
Эти производные можно найти следующим образом:
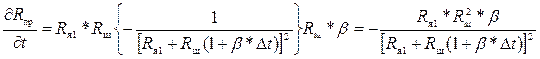 ;
;
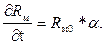 (3.9)
(3.9)
Для нахождения значения t, при котором имеет место наибольшая разность 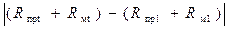 , необходимо использовать равенство (3.8):
, необходимо использовать равенство (3.8):
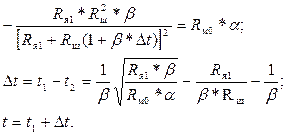 (3.10)
(3.10)
Погрешность компенсации равна
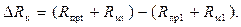 (3.12)
(3.12)
На эту погрешность вторичный прибор будет реагировать как на уменьшение сопротивления электродной ячейки на значение ΔRя:
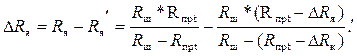 (3.13)
(3.13)
Такое уменьшение сопротивления электродной ячейки эквивалентно увеличению удельной электропроводности раствора на значение
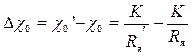 . (3.14)
. (3.14)
Абсолютная ΔС и относительная δ погрешности прибора, отградуированного в процентах концентрации анализируемого компонента, находятся по заданной зависимости удельной электропроводности χ0 от концентрации:
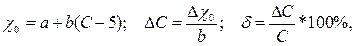
 (3.15)
(3.15)
где С – действительное значение концентрации.
Принцип действия потенциометрических анализаторов основан на измерении потенциала специального электрода, размещаемого непосредственно в анализируемом растворе (он должен быть электролитом). Этот потенциал зависит от концентрации некоторого определенного иона в анализируемом растворе. Примером такого анализатора является рН-метр, измеряющий концентрацию в растворе водородных ионов Н+.
Электродный потенциал измеряется по ЭДС гальванического элемента (рисунок 3.2), составленного из измерительного (индикаторного) электрода 1 и сравнительного (вспомогательного, или опорного) электрода 2. Оба электрода погружены в гальваническую ячейку с анализируемым раствором. Потенциал измерительного электрода Е и изменяется при изменении концентрации определяемых ионов в растворе, а потенциал сравнительного электрода Е ср остается постоянным, т.к. не зависит от нее. ЭДС Е такого гальванического элемента определяется разностью потенциалов электродов:
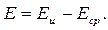 (3.16)
(3.16)
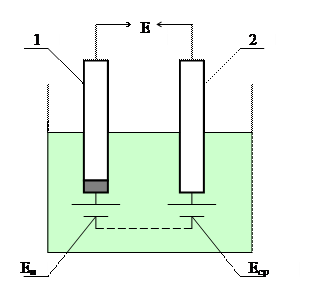
Рисунок 3.2 – Гальваническая ячейка потенциометрического анализатора
При измерении показателя рН разность потенциалов между измерительным и сравнительным электродами равна
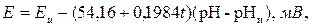 (3.17)
(3.17)
где t – температура раствора, 0С; Еи, рНи – координаты изопотенциальной точки (то есть точки, положение которой в координатах (Е; рН) не зависит от температуры). Она находится из условия равенства нулю частной производной функции Е = f(pH, t) по температуре.
Коэффициент преобразования электродной системы К равен
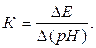 (3.18)
(3.18)
Для измерения ЭДС Е электродных систем используются устройства с очень высоким входным сопротивлением, т.к. показания милливольтметра U связаны со значением Е выражением
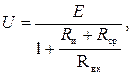 (3.19)
(3.19)
где Rи, Rср – сопротивления измерительного и сравнительного электродов; Rвх – входное сопротивление милливольтметра.
Задачи по теме 3
3-1. Возможна ли компенсация температурной погрешности ячейки кондуктометра (рисунок 3.1), если в качестве компенсатора использовать только медное сопротивление Rм?
Исходные данные для расчета:
- электроды измерительной ячейки не шунтированы;
- ячейка заполнена 5%-ным раствором KCl, удельная электропроводность которого при 20 0С составляет χ0 = 7,18 См/м, а температурный коэффициент β = 0,0201 К-1;
- постоянная ячейки К = 190 м-1;
- температурная компенсация должна осуществляться в диапазоне температур (20 – 40) 0С.
3-2. Определите сопротивление медного терморезистора Rм, обеспечивающего температурную компенсацию сопротивления ячейки (рисунок 3.1) при изменении температуры раствора в диапазоне (20 – 40) 0С.
Исходные данные для расчета:
- электроды измерительной ячейки шунтированы, причем сопротивление шунта Rш равно сопротивлению электродной ячейки Rя при температуре 20 0С;
- ячейка заполнена 5%-ным раствором KCl, удельная электропроводность которого при 20 0С составляет χ0 = 7,18 См/м, а среднее значение температурного коэффициента для указанного диапазона температур β = 0,0201 К-1;
- постоянная ячейки К = 190 м-1;
- температурный коэффициент сопротивления меди α = 0,00426 К-1.
3-3. Определите температуру, при которой будет иметь место наибольшая погрешность за счет неполной компенсации изменения сопротивления ячейки изменением сопротивления медного резистора (рисунок 3.1). Рассчитайте численное значение этой погрешности (абсолютной и относительной).
Исходные данные для расчета:
- электроды измерительной ячейки шунтированы, причем сопротивление шунта Rш равно сопротивлению электродной ячейки Rя при температуре 20 0С;
- температурная компенсация должна осуществляться в диапазоне температур (20 – 40) 0С;
- ячейка заполнена 5%-ным раствором KCl, удельная электропроводность которого при 20 0С составляет χ0 = 7,18 См/м, а среднее значение температурного коэффициента для указанного диапазона температур β = 0,0201 К-1;
- зависимость удельной электропроводности анализируемого раствора χ0 от концентрации С в рабочем диапазоне концентраций имеет вид χ0 = 7,18 + 1,38*(С-5);
- постоянная ячейки К = 190 м-1;
- температурный коэффициент сопротивления меди α = 0,00426 К-1.
3-4. Определите в общем виде зависимость абсолютной погрешности электродной системы (рисунок 3.2) рН -метра (в единицах рН) от температуры раствора. Определите численное значение абсолютной и относительной погрешности для случая, когда градуировка производилась при температуре t 1 = 20 0С, а действительное значение температуры t 2 = 35 0С. Действительное значение рН = 9. Уравнение электродной системы имеет вид
Е = Е и – (54,16 + 0,198* t) * (рН – рН и), мВ.
Координаты изопотенциальной точки: Е и = -203 мВ; рН и = 4,13.
3-5. Внутреннее сопротивление измерительного электрода Rи рН-метра равно 50 МОм, а электрода сравнения Rс - 20 кОм. ЭДС, развиваемая системой, составляет 500 мВ. Можно ли использовать для измерения этой ЭДС милливольтметр с диапазоном измерения (0 – 0,5) В и входным сопротивлением Rвх = 0,5 кОм?
3-6. Возможна ли компенсация температурной погрешности ячейки кондуктометра (рисунок 3.1), если в качестве компенсатора использовать только медное сопротивление Rм?
Исходные данные для расчета:
- электроды измерительной ячейки не шунтированы;
- ячейка заполнена 30%-ным раствором KCl, удельная электропроводность которого при 20 0С составляет χ0 = 39,07 См/м, а температурный коэффициент β = 0,0155 К-1;
- постоянная ячейки К = 210 м-1;
- температурная компенсация должна осуществляться в диапазоне температур (10 – 50) 0С.
3-7. Определите сопротивление медного терморезистора Rм, обеспечивающего температурную компенсацию сопротивления ячейки (рисунок 3.1) при изменении температуры раствора в диапазоне (15 – 45) 0С.
Исходные данные для расчета:
- электроды измерительной ячейки шунтированы, причем сопротивление шунта Rш равно сопротивлению электродной ячейки Rя при температуре 20 0С;
- ячейка заполнена 15%-ным раствором KCl, удельная электропроводность которого при 15 0С составляет χ0 = 20,91 См/м, а среднее значение температурного коэффициента для указанного диапазона температур β = 0,02 К-1;
- постоянная ячейки К = 200 м-1;
- температурный коэффициент сопротивления меди α = 0,00426 К-1.
3-8. Определите температуру, при которой будет иметь место наибольшая погрешность за счет неполной компенсации изменения сопротивления ячейки изменением сопротивления медного резистора (рисунок 3.1). Рассчитайте численное значение этой погрешности (абсолютной и относительной).
Исходные данные для расчета:
- электроды измерительной ячейки шунтированы, причем сопротивление шунта Rш равно сопротивлению электродной ячейки Rя при температуре 20 0С;
- температурная компенсация должна осуществляться в диапазоне температур (20 – 40) 0С;
- ячейка заполнена 30%-ным раствором KCl, удельная электропроводность которого при 20 0С составляет χ0 = 39,07 См/м, а среднее значение температурного коэффициента для указанного диапазона температур β = 0,0155 К-1;
- зависимость удельной электропроводности анализируемого раствора χ0 от концентрации С в рабочем диапазоне концентраций имеет вид χ0 = 7,26 + 1,33*(С-5);
- постоянная ячейки К = 180 м-1;
- температурный коэффициент сопротивления меди α = 0,00426 К-1.
3-9. Определите в общем виде зависимость абсолютной погрешности электродной системы (рисунок 3.2) рН -метра (в единицах рН) от температуры раствора. Определите численное значение абсолютной и относительной погрешности для случая, когда градуировка производилась при температуре t 1 = 20 0С, а действительное значение температуры t 2 = 40 0С. Действительное значение рН = 6. Уравнение электродной системы имеет вид
Е = Е и – (54,16 + 0,198* t) * (рН – рН и), мВ.
Координаты изопотенциальной точки: Е и = -203 мВ; рН и = 4,13.
3-10. Внутреннее сопротивление измерительного электрода Rи рН-метра равно 10 МОм, а электрода сравнения Rс - 20 кОм. ЭДС, развиваемая системой, составляет 500 мВ. Можно ли использовать для измерения этой ЭДС милливольтметр с диапазоном измерения (0 – 10) мВ и входным сопротивлением Rвх = 50 кОм?
3-11. Ячейка кондуктометра, измеряющего концентрацию NaCl, имеет форму цилиндра. Электроды диаметром 2 мм расположены на его торцах, расстояние между ними составляет 50 мм. Диапазон изменения концентрации С = 5 – 15 мг/л, удельная электропроводность раствора в этом диапазоне находится по зависимости χ0 = 182*С, мкСм/м. Номинальное значение концентрации 10 мг/л. Определите сопротивление шунта и медного термокомпенсирующего резистора для диапазона температур 20 – 40 0С. Температурный коэффициент NaCl β = 0,0231 К-1; ТКС меди α = 0,00426 К-1.
3-12. Возможна ли компенсация температурной погрешности ячейки кондуктометра (рисунок 3.1), если в качестве компенсатора использовать только медное сопротивление Rм?
Исходные данные для расчета:
- электроды измерительной ячейки не шунтированы;
- ячейка заполнена 1%-ным раствором KCl, удельная электропроводность которого при 20 0С составляет χ0 = 5,12 См/м, а температурный коэффициент β = 0,0201 К-1;
- постоянная ячейки К = 190 м-1;
- температурная компенсация должна осуществляться в диапазоне температур (20 – 40) 0С.
3-13. Определите сопротивление медного терморезистора Rм, обеспечивающего температурную компенсацию сопротивления ячейки (рисунок 3.1) при изменении температуры раствора в диапазоне (10 – 30) 0С.
Исходные данные для расчета:
- электроды измерительной ячейки шунтированы, причем сопротивление шунта Rш равно сопротивлению электродной ячейки Rя при температуре 10 0С;
- ячейка заполнена 1%-ным раствором KCl, удельная электропроводность которого при 20 0С составляет χ0 = 5,12 См/м, а среднее значение температурного коэффициента для указанного диапазона температур β = 0,0201 К-1;
- постоянная ячейки К = 190 м-1;
- температурный коэффициент сопротивления меди α = 0,00426 К-1.
3-14. Определите температуру, при которой будет иметь место наибольшая погрешность за счет неполной компенсации изменения сопротивления ячейки изменением сопротивления медного резистора (рисунок 3.1). Рассчитайте численное значение этой погрешности (абсолютной и относительной).
Исходные данные для расчета:
- электроды измерительной ячейки шунтированы, причем сопротивление шунта Rш равно сопротивлению электродной ячейки Rя при температуре 10 0С;
- температурная компенсация должна осуществляться в диапазоне температур (10 – 40) 0С;
- ячейка заполнена 1%-ным раствором KCl, удельная электропроводность которого при 20 0С составляет χ0 = 5,12 См/м, а среднее значение температурного коэффициента для указанного диапазона температур β = 0,0201 К-1;
- зависимость удельной электропроводности анализируемого раствора χ0 от концентрации С в рабочем диапазоне концентраций имеет вид χ0 = 5,12 + 1,38*(С-5);
- постоянная ячейки К = 190 м-1;
- температурный коэффициент сопротивления меди α = 0,00426 К-1.
3-15. Кондуктометрический солемер используется для измерения концентрации NaCl в растворе. Диапазон измеряемых концентраций 0 – 20%, постоянная ячейки 400 м-1. Зависимость удельной электропроводности раствора NaCl от концентрации в указанном диапазоне описывается уравнением χ0 = 6,26 + 1,08*(С-5). Определите погрешность, которая возникнет, если этим солемером измерять концентрацию KCl без градуировки. Текущее значение концентрации KCl составляет 9%, электропроводность связана с концентрацией соотношением χ0 = 7,01 + 1,14*(С-5).
3-16. Уравнение электродной системы рН -метра имеет вид
Е = Е и – (54,16 + 0,198* t) * (рН – рН и), мВ.
Координаты изопотенциальной точки: Е и = -203 мВ; рН и = 4,13.
Градуировка производилась при температуре t 1 = 20 0С. Определите действительное значение температуры, если при измерении раствора с рН = 6 прибор показал значение рН = 6,35.
3-17. Рассчитать потенциал стеклянного электрода в растворе с рН = 5,3 (при 20 °С) по отношению к хлорсеребряному электроду, если при рН = 3,38 (изопотенциальная точка) потенциал этого электрода по отношению к хлорсеребряному электроду равен —33 мВ.
3-18. Рассчитать рН раствора, если потенциал стеклянного электрода по отношению к хлорсеребряному электроду (при 20 °С) равен —133 мВ. Потенциал стеклянного электрода по отношению к хлорсеребряному при рН = 3,28 (изопотенциальная точка) равен —33 мВ.
3-19. Рассчитать рН раствора, если показания рН -метра, калиброванного по хлорсеребряному электроду, при использовании каломелевого насыщенного электрода составляют 5,0. Для хлорсеребряного электрода Е0 = 201 мВ, для каломелевого Е0 = 247 мВ.
3-20. Рассчитать потенциал стеклянного электрода в растворе при рН = 5,0 по отношению к хлорсеребряному электроду. Е0 стеклянного электрода при 20 °С составляет +358 мВ, Е0 хлорсеребряного электрода при этой же температуре равен +201 мВ.






