ќдним из многообразных физических свойств растворов электро≠литов €вл€етс€ электрическа€ проводимость, т.е. их способность проводить электрический ток под действием внешнего электрического пол€.
Ёта способность электролитов объ€сн€етс€ наличием ионов, несущих положительные и отрицательные зар€ды, которые в отсутствии внешнего электрического пол€ движутс€ беспор€дочно, все направлени€ перемещени€ ионов €вл€ютс€ равноверо€тными. ѕри нало≠жении внешнего электрического пол€ движение ионов становитс€ упо≠р€доченным. атионы двигаютс€ по направлению к катоду, а анионы в про≠тивоположном направлении - к аноду.
“ак как перенос электричества в растворах осуществл€етс€ за счет движени€ ионов, то количество электричества, переносимое через раствор, зависит от р€да факторов: от природы вещества (сильный или слабый электролит); концентрации ионов; температуры и других параметров.
ћерой электрической проводимости L растворов электролитов €вл€етс€ количество электричества, выраженное в кулонах, которое проходит через раствор за единицу времени. —ила тока, возникающего при движении ионов в растворе электролита под вли€нием внешнего электрического пол€, будет определ€тьс€ уравнением:
I = L × E
где I - сила тока, L - электрическа€ проводимость раствора, ≈ - напр€женность внешнего электрического пол€.
— другой стороны, согласно закону ќма, сила тока равна:

где R - сопротивление раствора.
ѕри сравнении этих двух уравнений получим:

“аким образом, электрическую проводимость раствора электроли≠та можно характеризовать как величину, обратную сопротивлению.
—опротивление электролита зависит от длины проводника ( ), площади поперечного сечени€ (S) и удельного сопротивлени€ (r):
), площади поперечного сечени€ (S) и удельного сопротивлени€ (r):

≈сли мы подставим значение R в уравнение электрической про≠водимости, то получим:


 где: величина, обратна€ удельному сопротивлению 1/r =, называетс€ удельной электрической проводимостью. ќбозначаетс€ она греческой буквой
где: величина, обратна€ удельному сопротивлению 1/r =, называетс€ удельной электрической проводимостью. ќбозначаетс€ она греческой буквой
(Ђкаппаї). ¬ результате получим уравнение:
 L = ×
L = × 
 ѕри условии, что S = 1 м2 и l = 1 м, электрическа€ проводимость раствора электролита будет равна удельной электрической проводимости:
ѕри условии, что S = 1 м2 и l = 1 м, электрическа€ проводимость раствора электролита будет равна удельной электрической проводимости:
L =
”дельна€ электрическа€ проводимость - это электрическа€ проводимость раст≠вора электролита, помещенного между электродами площадью поперечного сечени€ 1 м2, отсто€щими друг от друга на 1 м. “о есть удельной электрической проводимостью называетс€ электрическа€ проводимость 1 м3 данного электролита. ¬ системе —» единицей электрической проводимости €вл€етс€ Cименс (—м), следовательно, удельную электрическую проводимость растворов выражают в —м / м.
 “ак как переносчиками электрических зар€дов в растворах €в≠л€ютс€ ионы, то электрическа€ проводимость раствора будет тем больше, чем больше концентраци€ ионов, чем быстрее они дви≠жутс€ в электричес≠ком поле и чем больше их валентность. — уве≠личением концентрации удельна€ электрическа€ проводимость увеличи≠ваетс€, достигает максимального значени€, а при очень больших концентраци€х (пор€дка 10 моль/л и выше)
“ак как переносчиками электрических зар€дов в растворах €в≠л€ютс€ ионы, то электрическа€ проводимость раствора будет тем больше, чем больше концентраци€ ионов, чем быстрее они дви≠жутс€ в электричес≠ком поле и чем больше их валентность. — уве≠личением концентрации удельна€ электрическа€ проводимость увеличи≠ваетс€, достигает максимального значени€, а при очень больших концентраци€х (пор€дка 10 моль/л и выше)
|
|
|
начинает умень≠шатьс€ (рис.2).
 “ака€ зависимость четко выражена дл€ сильных электролитов и в меньшей степени дл€ слабых электролитов. Ќаличие максимумов на кривых объ€сн€етс€ тем, что в разбавленных растворах сильных электролитов скорость движени€ ионов почти не зависит от концентрации и растет пр€мо пропорционально числу ионов, которое увеличиваетс€ с концентрацией. ѕри достижении определенной концентрации в растворах сильных электролитов скорость движени€ ионов уменьшаетс€ из-за по€влени€ ионных атмосфер, вследствие чего уменьшаетс€ скорость движени€ иона, в результате чего удельна€ электрическа€ проводимость также уменьшаетс€.
“ака€ зависимость четко выражена дл€ сильных электролитов и в меньшей степени дл€ слабых электролитов. Ќаличие максимумов на кривых объ€сн€етс€ тем, что в разбавленных растворах сильных электролитов скорость движени€ ионов почти не зависит от концентрации и растет пр€мо пропорционально числу ионов, которое увеличиваетс€ с концентрацией. ѕри достижении определенной концентрации в растворах сильных электролитов скорость движени€ ионов уменьшаетс€ из-за по€влени€ ионных атмосфер, вследствие чего уменьшаетс€ скорость движени€ иона, в результате чего удельна€ электрическа€ проводимость также уменьшаетс€.

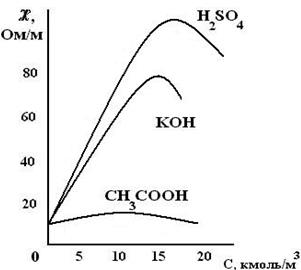 ƒл€ растворов слабых электролитов умень≠шение удельной электрической проводимости с ростом концентрации объ€сн€етс€ уменьшением степени дис≠социации. ¬ результате количество образовав≠шихс€ ионов в растворе слабого электролита будет возрастать в меньшей сте≠пени, чем аналитическа€ концентраци€ раствора.
ƒл€ растворов слабых электролитов умень≠шение удельной электрической проводимости с ростом концентрации объ€сн€етс€ уменьшением степени дис≠социации. ¬ результате количество образовав≠шихс€ ионов в растворе слабого электролита будет возрастать в меньшей сте≠пени, чем аналитическа€ концентраци€ раствора.
 |
–ис.2. «ависимость раство≠ров сильных и сла≠бых растворов электро≠литов от —.
ѕоскольку перенос электричества в растворах осуществл€етс€ за счет движени€ ионов, следова≠тельно, его количество зависит от скорости движени€ ионов, ко≠тора€ обратно пропорциональна их ионному радиусу.
“ак как ионы в водной среде гидратируютс€, то необходимо учитывать гидратированный Ђэффективныйї радиус иона, а не кристаллохимический.
Ёлектропроводность растворов щелочных металлов:
кристаллохимический радиус иона
 Li+ Na+ K+ Rb+ Cs+
Li+ Na+ K+ Rb+ Cs+
 радиус гидратированного иона
радиус гидратированного иона
јбсолютна€ скорость движени€ иона (U) Ц путь в метрах, пройденный ионом за 1 секунду при напр€женности электрического пол€ в 1 ¬ольт. –азмерность абсолютной скорости м2׬-1×с-1. Ёти скорости в обычных услови€х очень малы и составл€ют величины пор€дка 10-7Е 10-8 м2׬-1×с-1 Ќезначительна€ скорость ионов объ€сн€етс€ их высокой гидратацией и сопротивлением среды. ќбращают на себ€ внимание большие значени€ скоростей ионов водорода (33,6×10-8) и гидроксила (18,7×10-8) в воде по сравнению с другими ионами, что объ€сн€етс€ эстафетным перемещением этих ионов.
ѕротоны проход€т не весь путь до катода, а только рассто€ние между молекулами воды, т.е. как бы передаютс€ по эстафете от одной молекулы воды до другой. —редн€€ продолжительность жизни иона гидроксони€ (Ќ3ќ+) составл€ет 10-11сек. Ѕольша€ скорость движени€ ќЌ- - ионов объ€сн€етс€ тем же механизмом, однако при этом протон передаетс€ от молекулы воды к гидроксил-ионам. ¬ результате процесс выгл€дит как перемещение ќЌ- - ионов к аноду.
ƒл€ оценки количества электричества, переносимого через раствор катионами и анионами в отдельности, используетс€ пон€тие электролитической подвижности ионов - катиона ( к) и аниона (
к) и аниона ( а):
а):
 к = Uк F
к = Uк F
 а = Ua F,
а = Ua F,
где F Ц число ‘араде€ = 96500 кул.
¬ли€ние зар€да иона на удельную электрическую проводимость состоит в том, что, чем выше зар€д иона, тем больше электричества он перено≠сит с одного электрода на другой. ¬ таблице 10 даютс€  к и
к и  а некоторых ионов.
а некоторых ионов.
“аблица 10
ѕодвижности ионов (  ∞) при бесконечном разведении (катионы)
∞) при бесконечном разведении (катионы)
| ионы | температура, º— | ионы | температура, º— | ||||||
| Ќ+(Ќ2ќ) | 225,0 | 315,0 | 349,7 | 637,0 | ⅓Se+++ | - | - | 64,7 | - |
| Li+ | 19,1 | 33,4 | 38,7 | 120,0 | ⅓Ce+++ | - | - | 67,0 | - |
| Nа+ | 25,9 | 43,5 | 50,1 | 150,0 | ⅓Cr+++ | - | - | 67,0 | - |
| + | 40,3 | 64,6 | 73,5 | 200,0 | ½Mn++ | 27,0 | 44,0 | 53,5 | - |
| Rb+ | - | 67,5 | 77,5 | - | ½Fe++ | 28,0 | 44,0 | 53,5 | - |
| Cs+ | 44,0 | 68,0 | 76,8 | 200,0 | ⅓Fe+++ | - | - | 68,0 | - |
| NH4+ | 40,3 | 64,0 | 73,7 | 184,0 | ½Co++ | 28,0 | 45,0 | 54,0 | - |
| ½¬е++ | - | - | 45,0 | - | ½Ni++ | 28,0 | 45,0 | 54,0 | - |
| ½Mg++ | 28,5 | 45,0 | 53,1 | 170,0 | ½Cu++ | 20,0 | 45,0 | 56,0 | - |
| ½Ca++ | 30,8 | 51,0 | 59,5 | 187,0 | Ag+ | 33,0 | 54,0 | 61,9 | 180,0 |
| ½Sr++ | 31,0 | 51,0 | 59,5 | - | ½Zn++ | 28,0 | 45,0 | 53,5 | - |
| ½¬а++ | 33,6 | 55,0 | 63,7 | 200,0 | ½Cd++ | 28,0 | 45,0 | 54,0 | - |
| ½Ra++ | 33,0 | 56,6 | 66,8 | - | Tl+ | 43,0 | 66,0 | 74,9 | - |
| ⅓Al+++ | 29,0 | - | 63,0 | - | ½Rb++ | 38,0 | 60,0 | 70,0 | - |
јнионы
|
|
|
| ионы | температура, º— | ионы | температура, º— | ||||||
| OH- | 105,0 | 174,0 | 200,0 | 446,0 | ½SeO42- | - | 65,0 | 75,7 | - |
| F- | - | 46,6 | 55,4 | - | N3- | - | - | 69,5 | - |
| Cl- | 41,4 | 65,5 | 76,3 | 207,0 | NO2- | 44,0 | 59,0 | 72,0 | 189,0 |
| ClO2- | - | - | 52,0 | - | NO3- | 40,2 | 61,7 | 71,4 | - |
| ClO3- | 36,0 | 55,0 | 64,0 | 172,0 | NCO- | - | 54,8 | 64,6 | - |
| ClO4- | 37,3 | 59,1 | 68,0 | 179,0 | PO4H2- | - | 28,0 | 36,0 | - |
| Br- | 41,3 | 67,6 | 78,4 | - | ½PO4H2- | - | - | 57,0 | - |
| BrO3- | 31,0 | 49,0 | 56,0 | 155,0 | AsO4H2- | - | - | 34,0 | - |
| J- | 42,0 | 66,5 | 76,9 | - | CO3H- | - | - | 44,5 | - |
| JO3- | 21,0 | 33,9 | 41,0 | 127,0 | ½ CO32- | 36,0 | 60,5 | 72,0 | - |
| JO4- | - | 49,0 | 55,6 | - | CN- | - | - | 78,0 | - |
| SH- | 40,0 | 57,0 | 65,0 | - | ½CrO42- | 42,0 | 72,0 | 85,0 | - |
| SO3H- | 27,0 | - | 50,0 | - | MnO42- | 36,0 | 53,0 | 62,8 | - |
| ½ SO32- | - | - | 72,0 | - | HCOO- | - | 47,0 | - | - |
| ½ SO42- | 34,0 | 68,3 | 79,8 | 256,0 | CH3COO- | 20,0 | 34,0 | 41,0 | 130,0 |
| ½S2O82- | - | - | 86,0 | - | ½(C2O4)2- | 32,0 | 63,0 | - | - |
| SCN- | 41,7 | 56,6 | 66,5 | - |
— ростом температуры удельна€ электрическа€ проводимость растворов электролитов увеличиваетс€ в среднем на 2 % на каждый градус. ”величение электрической проводимости с ростом температуры объ€сн€етс€ уменьшением в€зкости воды, а также ростом кинетической энергии ионов, т.е. скорости их движени€.
 “ак как удельна€ электрическа€ проводимость раствора электролита определ€етс€ количеством ионов между электродами с
“ак как удельна€ электрическа€ проводимость раствора электролита определ€етс€ количеством ионов между электродами с  = 1 м и S = 1 м2 и скоростью этих ионов, то
= 1 м и S = 1 м2 и скоростью этих ионов, то
= —к Uk F + Ca Ua F = Ci ( k +
k +  a),
a),
где —i - ионна€ концентраци€ в 1 м3 раствора,  k и
k и  a Ц электролитические подвижности катиона и аниона.
a Ц электролитические подвижности катиона и аниона.
 онцентрацию раствора (—) обычно выражают на 1 литр, тогда
онцентрацию раствора (—) обычно выражают на 1 литр, тогда
1000 = —i ( k +
k +  a)
a)
Ёто уравнение называетс€ основным уравнением электрической проводимости.
¬ слабых электролитах ионна€ концентраци€ (Ci) св€зана с ана≠литической концентрацией уравнением:
Ci = C a,
где a - степень электролитической диссоциации.
 “огда основное уравнение электрической проводимости дл€ слабых электролитов будет иметь вид:
“огда основное уравнение электрической проводимости дл€ слабых электролитов будет иметь вид:
1000 = — a ( k +
k +  a)
a)
—тепень электролитической диссоциации показывает, кака€ часть молекул электролита в растворе распалась на ионы:
a = 
«начение a измер€етс€ в пределах от 0 до 1. «ависит от природы электролита, природы растворител€, температуры раствора и степени его разбавлени€. ” большинства электролитов степень диссоциации по мере увеличени€ температуры увеличиваетс€, а у некоторых (NH4OH, —Ќ3—ќќЌ) достигает максимума, а затем уменьшаетс€ в св€зи с уменьшением диэлектрической посто€нной растворител€, что благопри€тствует образованию молекул из ионов.
»звестно, чем больше диэлектрическа€ посто€нна€ растворител€, тем сильнее выражен процесс диссоциации растворенного в нем вещества. — разбавлением раствора электролита веро€тность взаимодействи€ ионов в растворе уменьшаетс€, степень электролитической диссоциации увеличиваетс€.
|
|
|
¬ сильных электролитах, где молекулы растворенного вещества полностью диссоциированы на ионы, ионна€ концентраци€ св€зана с аналитической концентрацией уравнением:
Ci = C fэ ,
 где fэ - коэффициент электрической проводимости, отражающий меру электростатического взаимодействи€ и гидратации ионов в растворе. ѕо своему физическому смыслу fэ соответствует fa -коэффициенту активности. fэ увеличиваетс€ при разбавлении раствора и достигает 1 при максимальном разбавлении, когда силы взаимодействи€ между ионами приближаютс€ к нулю. ƒл€ разбавленных (—£ 0.1) растворов электролитов fэї 1. ѕоэтому при работе с биологическими растворами принимают fэ =1. ƒл€ сильных электролитов основное уравнение электропроводности имеет вид:
где fэ - коэффициент электрической проводимости, отражающий меру электростатического взаимодействи€ и гидратации ионов в растворе. ѕо своему физическому смыслу fэ соответствует fa -коэффициенту активности. fэ увеличиваетс€ при разбавлении раствора и достигает 1 при максимальном разбавлении, когда силы взаимодействи€ между ионами приближаютс€ к нулю. ƒл€ разбавленных (—£ 0.1) растворов электролитов fэї 1. ѕоэтому при работе с биологическими растворами принимают fэ =1. ƒл€ сильных электролитов основное уравнение электропроводности имеет вид:
1000 = —i fэ ( k +
k +  a)
a)
ѕоскольку удельна€ электрическа€ проводимость зависит от многих факторов, и на еЄ основе нельз€ сделать какие-либо выводы о вли€нии на величину проводимости электролитов концентрации ионов, а также силы их взаимодействи€, Ћенц ввел пон€тие мол€рной или эквивалентной электрической проводимости.
ћол€рной электрической проводимостью λv называетс€ электрическа€ проводимость столба раствора, содержащего 1 кмоль (г×экв) электролита, заключен≠ного между электродами, расположенными на рассто€нии 1 м друг от друга. ћол€рную электрическую проводимость обозначают греческой буквой l (Ђл€мбдаї). „тобы показать, к какому разбавлению раствора относитс€ мол€рна€ электрическа€ проводимость, возле буквы l ставитс€ индекс (v), обозначающий разбавление в литрах (V = 1/—), т.е. количество литров, содержащее 1 кмоль электролита, - lv.
 ‘ормула, св€зывающа€ мол€рную электрическую проводимость с удельной имеет вид:
‘ормула, св€зывающа€ мол€рную электрическую проводимость с удельной имеет вид:
λv = 1000 V
 ќтсюда:
ќтсюда:
 λv = 1000
λv = 1000
— 
–азмерность lv - —м×м2/кмоль.
ѕоследний множитель часто опускаетс€ и мол€рную электрическую проводимость выражают в —м×м2, име€ в виду 1 кмоль растворенного вещества.
ћол€рна€ электрическа€ проводимость у сильных и слабых электро≠литов возрастает с увеличением разбавлени€ и достигает предельного значени€, которое называетс€ мол€рной электрической проводимостью при бесконечном разбавлении и обозначаетс€ l¥.
ћол€рна€ электрическа€ проводимость обусловлена при любом раз≠бавлении одинаковым количеством электролита, а именно 1 кмоль. ≈е возрастание с разбавлением дл€ слабых электролитов объ€сн€ет≠с€ тем, что при этом увеличиваетс€ степень диссоциации, т.е. увеличиваетс€ количество свободных ионов, перенос€щих электричество.
ѕри достаточно большом разбавлении наступает полна€ диссоциаци€ раствора (a = 1), в этот момент lv достигает максимального зна≠чени€, равного l¥, и дальнейшее разбавление не измен€ет данную величину, так как число ионов в растворе остаетс€ неизменным.

–ис.3. ¬ли€ние разбавлени€ раствора на мол€рную электрическую проводимость.
»з рисунка 3 видно, что мол€рна€ электрическа€ проводимость растворов сильных электролитов быстро увеличиваетс€ и уже при умеренном разбавлении почти достигает предела. ѕри разбавлении электролита увеличиваетс€ объем раствора, соответственно увеличиваетс€ рассто€ние между ионами. ¬ определенный момент ионы достигают максимальной скорости движени€. ѕосле этого с увеличением разбавлени€ скорость движени€ остаетс€ неизменной, а мол€рна€ электрическа€ проводимость переходит в мол€рную электрическую проводимость при бесконечном разбавлении (l¥).
–астворы слабых электроли≠тов ведут себ€ иначе. »х мол€рна€ электрическа€ проводимость до разбавлени€ близка к нулю и затем медленно возрас≠тает с увеличением разбавлени€, так как увеличиваетс€ степень диссоциации.
|
|
|
Ќа основании изложенного выше:
l¥ = 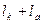
—ледовательно, мол€рна€ электрическа€ проводимость электролита при бесконечном разбавлении равна сумме электролитических подвижностей катиона и аниона. ¬ этом заключаетс€ закон ольрауша (закон аддитивности) или закон независимого перемещени€ ионов.
ћол€рна€ электрическа€ проводимость также зависит от температуры и увеличиваетс€ на 2 - 2,5 % с увеличением температуры на гра≠дус.

 »спользу€ основное уравнение электрической проводимости дл€ слабых электролитов и закон ольрауша, можно рассчитать степень электролитической диссоциации:
»спользу€ основное уравнение электрической проводимости дл€ слабых электролитов и закон ольрауша, можно рассчитать степень электролитической диссоциации:
 1000 = — a (
1000 = — a ( k +
k +  a)
a)  α = 1000 /—(
α = 1000 /—( k +
k +  a),
a),
где 1000 /— = λv, ( k +
k +  a) = l¥, тогда
a) = l¥, тогда
α = λv / l¥ (уравнение јррениуса)
 ¬ случае сильных электролитов аналогично можно рассчитать коэффициент электрической проводимости:
¬ случае сильных электролитов аналогично можно рассчитать коэффициент электрической проводимости:
fэ = 1000 /—( k +
k +  a)= λv / l¥
a)= λv / l¥
—тепень диссоциации зависит от концентрации и поэтому непригодна дл€ количественной оценки силы электролита. ћерой силы сла≠бого электролита €вл€етс€ константа электролитической диссоциа≠ции, величина которой дл€ данного электролита посто€нна при любых концентраци€х. онстанта электролитической диссоциации определ€етс€ природой электролита и мен€етс€ лишь с изменением температуры. „ем константа электролита меньше, тем слабее данный электролит:

ѕриведенна€ формула €вл€етс€ аналитическим выражением закона разбавлени€ ќствальда.






