’»ћ»я
ћетодические указани€ к практическим зан€ти€м
и дл€ самосто€тельной подготовки студентов всех
специальностей дневной и заочной форм обучени€
—троение атома и химическа€ св€зь

ћогилев 2011
”ƒ 54
ЅЅ 24
’ 46
–екомендовано к опубликованию
учебно-методическим управлением
√” ¬ѕќ ЂЅелорусско-–оссийский университетї
ќдобрено кафедрой Ђ“ехнологии металловї Ђї ма€ 2011 г., протокол є
—оставители: канд. хим. наук, доцент ». ћ. Ћужанска€
канд. биол. наук, ст. преподаватель ». ј. Ћисова€
–ецензент ст. преподаватель ¬.‘. ѕацей
¬ методических указани€х рассмотрены современные представлени€ о строении атома, структура периодической системы элементов, даетс€ объ€снение свойств химических элементов в зависимости от их положени€ в периодической системе. ѕредставлены основные виды химической св€зи и механизмы их образовани€. ƒаны примеры составлени€ электронных конфигураций атомов и схемы образовани€ химических соединенийэ.
’»ћ»я
ќтветственный за выпуск ƒ. ». якубович
“ехнический редактор ј. “. „ервинска€
омпьютерна€ верстка Ќ. ѕ. ѕолевнича€
ѕодписано в печать. ‘ормат 60x84/16. Ѕумага офсетна€. √арнитура “аймс.
ѕечать трафаретна€. ”сл.- печ. л.. ”ч.-изд. л.. “ираж 180 экз. «аказ є
»здатель и полиграфическое исполнение
√осударственное учреждение высшего профессионального образовани€
ЂЅелорусско-–оссийский университетї
Ћ» є 02330/375 от 29.06.2004 г.
212000, г. ћогилев, пр. ћира, 43
© √” ¬ѕќ ЂЅелорусско-–оссийский
университетї, 2011
1 ќсновные пон€ти€ химии
’ими€ Ч одна из важнейших и обширных областей естествознани€, наука о веществах, их свойствах, строении и превращени€х, происход€щих в результате химических реакций, а также фундаментальных законах, которым эти превращени€ подчин€ютс€.
¬ещество Ч вид материи, котора€ обладает массой поко€. —остоит из элементарных частиц: электронов, протонов, нейтронов, мезонов и др. ’ими€ изучает главным образом вещество, организованное в атомы, молекулы, ионы и радикалы. “акие вещества прин€то подраздел€ть на простые и сложные (химические соединени€).
1.1 ѕростые и сложные вещества. јллотропи€
ѕростые вещества образованы атомами одного химического элемента и поэтому €вл€ютс€ формой его существовани€ в свободном состо€нии, например, сера, железо, озон, алмаз, азот.
—ложные вещества образованы разными элементами и могут иметь состав посто€нный (стехиометрические соединени€ или дальтониды) или мен€ющийс€ в некоторых пределах (нестехиометрические соединени€ или бертоллиды).
’имический элемент Ч множество атомов с одинаковым зар€дом €дра, числом протонов, совпадающим с пор€дковым номером в ѕериодической системе элементов ћенделеева. аждый химический элемент имеет свое название и символ.
јтом Ч наименьша€ химически неделима€ часть химического элемента, €вл€юща€с€ носителем его свойств.
ѕон€тие простое вещество нельз€ отождествл€ть с пон€тием химический элемент. —войства химического элемента относ€тс€ к его отдельным атомам. —войства простого вещества: плотность, растворимость, температуры плавлени€ и кипени€ относ€тс€ к совокупности атомов. ќдин и тот же химический элемент может существовать в виде двух и более простых веществ, различных по строению и свойствам. Ёто €вление называетс€ аллотропией, а образующие вещества - аллотропными модификаци€ми или аллотропными формами.
|
|
|
’имический элемент кислород образует две аллотропные модификации: кислород и озон, элемент углерод образует четыре аллотропные модификации: алмаз, графит, карбин, фуллерен.
явление аллотропии вызываетс€ двум€ причинами: различным числом атомов в молекуле (например, кислород ќ2 и озон ќ3) либо образованием различных кристаллических форм (например, углерод образует аллотропные модификации, такие как алмаз, графит, карбин, фуллерен).
¬ структуре алмаза каждый атом углерода расположен в центре тетраэдра, вершинами которого служат четыре ближайших атома.
¬ кристаллической структуре графита атомы углерода формируют шестиугольные кольца, образующие, в свою очередь, прочную и стабильную сетку, похожую на пчелиные соты. —етки располагаютс€ друг над другом сло€ми, которые слабо св€заны между собой.
¬ молекуле карбина атомы углерода соединены в цепочки либо тройными и одинарными св€з€ми, либо двойными св€з€ми.
¬ фуллерене плоска€ сетка шестиугольников свернута и сшита в замкнутую сферу. јтомы углерода, образующие сферу, св€заны между собой сильной св€зью.
—ложные вещества состо€т не из простых веществ, а из химических элементов. “ак, водород и кислород, вход€щие в состав воды, содержатс€ в воде не в виде газообразных водорода и кислорода с их характерными свойствами, а в виде элементов водорода и кислорода.
¬ещества подраздел€ютс€ на вещества молекул€рного и немолекул€рного строени€.
¬ещества молекул€рного строени€ Ц это вещества, основной структурной единицей которых €вл€етс€ молекула.
¬ещества немолекул€рного строени€ Ц это вещества, основными структурными единицами которых €вл€ютс€ атомы или ионы.
ƒл€ отображени€ качественного и количественного состава вещества используетс€ формульна€ единица.
‘ормульна€ единица (‘≈) Ц реальна€ или условна€ частица, обозначаема€ химической формулой.
’имическа€ формула Ц условна€ запись состава вещества при помощи химических символов и индексов.
‘ормульной единицей вещества молекул€рного строени€ €вл€етс€ молекула.
ћолекула Ц электронейтральна€ частица вещества, представл€юща€ собой замкнутую совокупность конечного числа атомов, св€занных между собой силами ковалентной св€зи и образующих определенную структуру.
‘ормульной единицей простого вещества немолекул€рного строени€ €вл€етс€ атом. Ќапример, формульна€ единица кремни€ атом Si.
‘ормульной единицей сложного вещества немолекул€рного строени€ €вл€етс€ Ђусловна€ молекулаї. Ќапример, формульной единицей оксида кремни€ €вл€етс€ условна€ частица, состо€ща€ из одного атома кремни€ (Si) и двух атомов кислорода (ќ). ќна €вл€етс€ условной потому, что в кристалле оксида кремни€(IV) нет отдельных молекул SiO2, он состоит из множества атомов кремни€ и кислорода. Ќо весь кристалл можно условно разделить на группы, в каждой из которых будет один атом Si и два атома ќ. “аким образом, формульна€ единица оксида кремни€ (IV) Цусловна€, реально не существующа€ частица Ц SiO2.
|
|
|
≈сли вещество немолекул€рного строени€ образует ионную кристаллическую решетку, например хлорид натри€. ≈го формульной единицей будет условна€ частица, состо€ща€ из одного иона Na+ и одного иона Cl-. ќна €вл€етс€ условной потому, что в кристалле хлорида натри€ нет молекул NaCl, так как он состоит из ионов. Ќо весь этот кристалл можно разделить на группы ионов, в каждой из которых будет один ион Na+ и один ион Cl-. —ледовательно, формульной единицей хлорида натри€ €вл€етс€ условна€ частица, состо€ща€ из двух ионов Ц NaCl.
1.2 ќтносительна€ атомна€ масса
—овременные методы исследовани€ позвол€ют определить чрезвычайно малые массы атомов с большой точностью. “ак, например, масса атома водорода составл€ет 1,674 × 10-27 кг, углерода Ц 1,993 × 10-26 кг.
¬ химии традиционно используютс€ не абсолютные значени€ атомных масс, а относительные. ќтносительными они называютс€ потому, что вычисл€ютс€ по отношению к массе эталона. ¬ насто€щее врем€ в качестве эталона выбрана 1/12 часть абсолютной массы атома изотопа углерода 12— - атомна€ единица массы (сокращенно а.е.м.).
а.е.м. = ma(12C)/12 = 19.9272 · 10-27 кг/12 = 1,66· 10-27 кг = 1,66 ·10-24 г
ќтносительна€ атомна€ масса Ц безразмерна€ величина, равна€ отношению абсолютной массы данного атома к 1/12 части массы изотопа углерода 12—.
’имические элементы в природе представл€ют собой смесь изотопов с различной массовой долей. »сход€ из этого, под абсолютной массой атома химического элемента подразумеваетс€ средн€€ величина.
—редн€€ абсолютна€ масса атома элемента Ц масса атома элемента, выраженна€ в кг, вычисленна€ с учетом его изотопного состава.
ќтносительна€ атомна€ масса элемента (или просто атомна€ масса) Ц безразмерна€ величина, равна€ отношению средней абсолютной массы атома элемента к 1/12 части массы изотопа 12—.
јтомные массы элементов обозначают јr, где индекс r Ц начальна€ буква английского слова relative Ц относительный. «аписи Ar(H), Ar(O), Ar(C ) Ц это относительна€ атомна€ масса водорода, относительна€ атомна€ масса кислорода, относительна€ атомна€ масса углерода соответственно.
1.3 ќтносительна€ молекул€рна€ масса
ќтносительной молекул€рной массой вещества (ћr) называетс€ величина, равна€ отношению массы молекулы вещества к 1/12 массы атома углерода 12—.
ћолекул€рна€ масса численно равна сумме относительных атомных масс всех атомов, вход€щих в состав молекулы вещества.
ќтносительна€ молекул€рна€ масса показывает, во сколько раз масса молекулы данного вещества больше 1/12 массы атома 12 —. “ак, молекул€рна€ масса кислорода Mr(O2 ) равна 32. Ёто означает, что масса молекулы кислорода в 32раза больше, чем 1/12 массы атома 12C.
сложным веществам немолекул€рного строени€ нельз€ применить пон€тие Ђотносительна€ молекул€рна€ массаї. ѕоскольку структурными единицами таких веществ €вл€ютс€ не молекулы, а условные формульные единицы, к ним применим термин Ђотносительна€ формульна€ массаї(ћfr).
ќтносительна€ формульна€ масса Ц величина, равна€ отношению массы одной формульной единицы вещества к 1/12 части массы изотопа 12—.
 1.4 ћоль. ћол€рна€ масса
1.4 ћоль. ћол€рна€ масса
¬ химических процессах участвуют мельчайшие частицы Ц молекулы, атомы, ионы, электроны. „исло таких частиц даже в малой порции вещества очень велико. ѕоэтому, чтобы избежать математических операций с большими числами, дл€ характеристики количества вещества, участвующего в химической реакции, используетс€ специальна€ единица Ц моль.
ћоль Ц количество вещества, содержащее в своем составе столько атомов, молекул, ионов, электронов или других структурных единиц, сколько атомов содержитс€ в 0,012 кг углерода 12—.
„исло атомов в 0,012 кг углерода, или в 1 моль называетс€ числом јвогадро (NA) и составл€ет 6,02 · 1023.
»сход€ из этого, можно сказать, что моль Ц это количество вещества, которое содержит 6,02 × 1023 структурных единиц (молекул, атомов, ионов, электронов и др.)
|
|
|
ѕримен€€ пон€тие моль, необходимо в каждом конкретном случае точно указать, какие именно структурные единицы имеютс€ в виду. Ќапример, моль атомов Ќ, моль молекулы H2, моль ионов H +.
ћасса одного мол€ вещества называетс€ мол€рной массой вещества (M).
ћасса вещества (m) численно равна произведению его количества (n) на мол€рную массу: 
ѕоскольку в одном моле любого вещества содержитс€ одинаковое количество структурных единиц, то мол€рна€ масса вещества пропорциональна массе соответствующей структурной единицы, т. е. относительной молекул€рной массе (ћr):
ћ = · ћr
= 1, т. к. дл€ углерода ћ r = 12 а.е.м., а мол€рна€ масса равна 12 (по определению пон€ти€ мол€), следовательно, численные значени€
ћ (г/моль) = ћ r.
ќтсюда следует, что мол€рна€ масса вещества, выраженна€ в граммах, имеет то же численное значение, что и его относительна€ молекул€рна€ масса.
1.5 Ёквивалент. ‘актор эквивалентности. ћол€рна€ масса эквивалента
Ёквивалент(Ё) Ц реальна€ или условна€ частица вещества, котора€ может замещать, присоедин€ть или быть каким-либо другим способом эквивалентна (то есть равноценна) одному атому или иону водорода в обменных реакци€х или одному электрону в окислительно-восстановительных реакци€х.
„астица вещества, называема€ эквивалентом, может быть равна или в целое число раз меньше формульной единицы, соответствующей данному веществу.
» так же, как состав молекул, атомов или ионов, состав эквивалента выражаетс€ с помощью химических знаков и формул.
ƒл€ того чтобы определить состав эквивалента вещества и правильно записать его химическую формулу, надо исходить из конкретной реакции, в которой участвует данное вещество.
ѕриведены несколько примеров определени€ формулы эквивалента.
¬ обменной реакции
KOH + HCl = KCl + H2O; (1)
K+ + OHЦ+ H+ + ClЦ = K+ + ClЦ + H2O;
H+ + OHЦ = H2O
с одним ионом водорода реагирует один ион гидроксила.
—огласно определению эквивалента, Ё(ќЌЦ) = ќЌЦ, а эквивалент гидроксида кали€ будет соответственно равен формульной единице ќЌ:
Ё( ќЌ) = ќЌ.
¬ обменной реакции
Ca(OH)2 + 2HCl = CaCl2 + 2H2O (2)
Ca2+ + 2OHЦ + 2H+ + 2ClЦ = Ca2+ + 2ClЦ = 2H2O
один ион водорода эквивалентен 1/2 иона 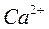 , одному иону OHЦ и одному иону ClЦ.
, одному иону OHЦ и одному иону ClЦ.
—ледовательно, Ё(ClЦ) = ClЦ; Ё(—а2+) = 1/2—а2+; Ё(ќЌЦ) = ќЌЦ.
¬месте с тем, согласно молекул€рному уравнению, с одной молекулой гидроксида кальци€ взаимодействует две молекулы сол€ной кислоты, то есть два иона водорода. —ледовательно, один ион водорода потребуетс€ на взаимодействие с 1/2 —а(ќЌ)2. “огда по определению эквивалентом гидроксида кальци€ €вл€етс€ частица, равна€  формульной единицы, то есть ½ —а(ќЌ)2.
формульной единицы, то есть ½ —а(ќЌ)2.  .
.
¬ реакции восстановлени€ катиона цинка
Zn2+ + 2e = Zn0 (3)
с одним ионом цинка взаимодействуют два электрона, следовательно, одному электрону эквивалентна 1/2 иона Zn2+ и Ё(Zn2+) = 1/2Zn2+.
¬ реакции
Fe3+ + e = Fe2+ (4)

ион Fe3+реагирует с одним электроном, и, соответственно,

¬ реакции
Fe3+ + 3e = Fe0 (5)
ион Fe 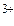 присоедин€ет три электрона, следовательно, Ё(Fe3+) = 1/3Fe3+.
присоедин€ет три электрона, следовательно, Ё(Fe3+) = 1/3Fe3+.
„исло, показывающее, кака€ часть формульной единицы вещества соответствует эквиваленту, называетс€ фактором эквивалентности (fэ).
ѕо реакции (1): fэ(OH 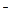 ) = 1; fэ( OH) = 1.
) = 1; fэ( OH) = 1.
ѕо реакции (2): fэ(OH 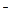 ) = 1; fэ((Cа2+) = 1/2; fэ (Cа(ќЌ)2) = 1/2.
) = 1; fэ((Cа2+) = 1/2; fэ (Cа(ќЌ)2) = 1/2.
ѕо реакции (3) fэ (Zn2+) = 1/2.
ѕо реакции (4) fэ (Fe 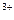 ) = 1.
) = 1.
ѕо реакции (5) fэ(Fe 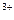 ) = 1/3.
) = 1/3.
“аким образом, сочета€ фактор эквивалентности и формульную единицу вещества, можно составить формулу эквивалента какой-либо частицы, где фактор эквивалентности записываетс€ как коэффициент перед формулой частицы:
fэ (формульна€ единица вещества) = эквивалент.
|
|
|
—ледует учитывать,что эквивалент одного и того же вещества мен€етс€ в зависимости от того, в какую реакцию он вступает. Ёквивалент элемента также может быть различным в зависимости от вида соединени€, в состав которого он входит.
‘актор эквивалентности химического элемента.
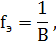
где B Ц валентность элемента в данном соединении.
Ќапример, в H2S Ц fэ(S) = 1/2, Ё(S) = 1/2; в NH 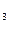 - fэ(N) = 1/3,
- fэ(N) = 1/3,
Ё(N) = 1/3N; в AlCl 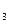 - fэ(Al) = 1/3, Ё(Al) = 1/3Al, fэ(Cl) = 1, Ё(Cl) = Cl.
- fэ(Al) = 1/3, Ё(Al) = 1/3Al, fэ(Cl) = 1, Ё(Cl) = Cl.
‘актор эквивалентности кислоты зависит от ее основности, котора€ определ€етс€ числом ионов водорода, замещающихс€ в реакции на атомы металла (n(H+)):
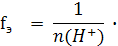
≈сли кислота многоосновна€, то fэ может принимать различные значени€. Ќапример, в реакции
H2SO4+ KOH = KHSO4 + H2O (6)
 серна€ кислота обменивает на металл один атом водорода, fэ(Ќ2SO4) = 1, Ё(H2SO4) = H2SO4.
серна€ кислота обменивает на металл один атом водорода, fэ(Ќ2SO4) = 1, Ё(H2SO4) = H2SO4.
¬ реакции
H2SO4 + 2KOH = K2SO4 +2H2O (7)
серна€ кислота обменивает на металл два атома водорода, т. е. ведет себ€ как двухосновна€ кислота, поэтому fэ(H2SO4) = 1/2, Ё(H2SO4) = 1/2 H2SO4.
‘актор эквивалентности основани€ зависит от кислотности основани€, котора€ определ€етс€ числом гидроксильных групп, обменивающихс€ в реакции на кислотный остаток (n(OH-):
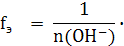
ƒл€ многокислотных оснований fэ Ц величина переменна€ и зависит от условий проведени€ реакции. Ќапример, в реакции
Al(OH)3 + 2HCl = Al(OH)2Cl + 2H2O (8)
гидроксид алюмини€ обменивает одну гидроксильную группу на кислотный остаток, поэтому fэ(Al(OH)3) = 1, Ё(Al(OH)3) = Al(OH)3.
¬ реакции
Al(OH)3 + 2HCl = Al(OH)Cl2 + 2H2O (9)
гидроксид алюмини€ обменивает две гидроксильные группы на кислотный остаток, поэтому fэ(Al(OH)3) = 1/2, Ё(Al(OH)3) = 1/2Al(OH)3.
¬ реакции
Al(OH)3 + 3HCl = AlCl3 + 3H2O (10)
гидроксид алюмини€ обменивает три гидроксильные группы на кислотный остаток, поэтому fэ(Al(OH)3) = 1/3, Ё(Al(OH)3) = 1/3Al(OH)3.
‘актор эквивалентности средней соли определ€етс€ формулой
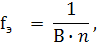
где ¬ Ц валентность метала,
n Ц число атомов металла.
Ќапример, fэ(Na2SO4) = 1/(1·2) = 1/2; fэ(Fe2SO4)3) = 1/(2·3) =1/6.
‘актор эквивалентности кислых и основных солей определ€етс€ исход€ из уравнени€ реакции с учетом того, что вещества взаимодействуют друг с другом в эквивалентных количествах.
B реакции
NaHSO4 +NaOH = Na2SO4 + H2O (11)
одна молекула гидросульфата натри€ взаимодействует с одним эквивалентом NaOH, следовательно, fэ(NaHSO4) = 1, Ё(NaHSO4) = NaHSO4.
¬ реакции
NaHSO4 + BaCl2 = BaSO4 + NaCl + HCl(12)
одна молекула гидросульфата натри€ взаимодействует с двум€ эквивалентами хлорида бари€, т.к. fэ(¬аCl2) = 1/2 и Ё(BaCl2) = 1/2BaCl2, следовательно, fэ(NaHSO4 ) также равен 1/2 и Ё(NaHSO4) = 1/2NaHSO4.
¬ реакции
Al(OH)Cl2 + HCl = AlCl3 + H2O (13)
одна молекула дихлорида гидроксоалюмини€ взаимодействует с одним эквивалентом HCl, поэтому fэ(Al(OH)Cl2) = 1, Ё(Al(OH)Cl2) = Al(OH)Cl2.
¬ реакции
Al(OH)Cl2 + 2NaOH= Al(OH)3 + 2NaCl (14)
одна молекула дихлорида гидроксоалюмини€ взаимодействует с двум€ эквивалентами NaќЌ (fэ(NaOH) = 1), следовательно, fэ(AlOHCl2) = 1/2, Ё(AlOHCl2) = 1/2 AlOHCl2.
¬ реакции
Al(OH)Cl2 + Na3PO4 = AlPO4 + 2NaCl= Na(OH) (15)
одна молекула дихлорида гидроксоалюмини€ взаимодействует с трем€ эквивалентами Na3PO4(fэ(Na3PO4) = 1/3), поэтому fэ(AlOHCl2) = 1/3, Ё(AlOHCl2) = 1/3AlOHCl2.
‘актор эквивалентности оксидов, про€вл€ющих основные свойства, определ€етс€ по формуле
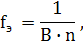
где ¬ Ц валентность металла,
n Ц число атомов металла в оксиде.
Ќапример: CaO fэ(—aO) = 1/2, Ё(CaO) = 1/2 CaO;
Na2O fэ(Na2O) = 1/2, Ё(Na2O) = 1/2Na2O;
Al2O3 fэ(Al2O3) = 1/6, Ё(Al2O3) = 1/6 Al2O3.
‘актор эквивалентности оксидов, про€вл€ющих кислотные свойства, определ€етс€ исход€ из уравнени€ реакции.
¬ реакции
SO3+ 2NaOH= Na2SO4+ H2O(16) одна молекула оксида серы (VI) взаимодействует с двум€ эквивалентами гидроксида натри€ (fэ(NaOH) = 1 ), cледовательно, fэ(SO3) = 1/2, Ё(SO3) = 1/2SO3.
¬ реакции
Al2O3 + 2NaOH = 2NaAlO2 + H2O (17)
одна молекула оксида алюмини€ взаимодействует с двум€ эквивалентами гидроксида натри€, поэтому fэ(Al2O3) равен 1/2, Ё(Al2O3) = 1/2 Al2O3.
“аким образом, на основании всех вышеприведенных примеров можно сделать вывод, что фактор эквивалентности любого вещества равен единице, деленной на число образующихс€ либо перестраивающихс€ св€зей.
ƒл€ эквивалента справедливы все пон€ти€, характеризующие структурные единицы вещества, в том числе количество вещества и мол€рна€ масса вещества.
оличество вещества эквивалентов измер€етс€ в мол€х.
ћоль эквивалентов Ц это количество вещества, которое соедин€етс€ с 1 молем атомов водорода или 1/2 мол€ атомов кислорода или замещает те же количества водорода в их соединени€х. Ќапример, в соединени€х HCl,H2S, NH3, CH4 моль эквивалентов хлора, серы, азота, углерода равен соответственно 1 моль Cl, 1/2 мол€ S, 1/3 мол€ N, 1/4 мол€ углерода.
|
|
|
ћол€рна€ масса эквивалента (ћэ) Ц это масса одного мол€ эквивалентов.
ƒл€ нахождени€ мол€рной массы эквивалентов химического элемента нужно мол€рную массу данного элемента умножить на фактор эквивалентности:
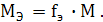
Ќапример, в соединени€х:
HCl Mэ(Cl) = fэ(Cl) · M(Cl) = 1· 35.5 г/моль;
NH3 Mэ(N) = fэ(N) · M(N) = 1/3· 14 = 4.67 г/моль;
H2S ћэ(S) = fэS) · Ms = 1/2 · 32 = 16 г/моль;
CH4 ћэ(C) = fэ · Mc = 1/4 · 12 = 3 г/моль.
ƒл€ кислот, оснований, средних солей и оксидов, про€вл€ющих основные свойства, мол€рна€ масса эквивалентов может быть рассчитана как сумма мол€рных масс эквивалентов, составл€ющих данное соединение ионов или элементов, если речь идет об оксидах.
Ќапример, в реакции (6) ћэ(H2SO4) равна:
ћэ(Ќ+) + ћэ(HSO4Ц) = fэ(H+)· M(H+) + fэ(HSO4Ц) · M(HSO4Ц) = 98 г/моль.
¬ реакции (7) ћэ(H2SO4) равна:
ћэ(Ќ+) + ћэ(SO42Ц) = fэ(H+) · M(H+) + fэ(SO42Ц) · M(SO42Ц) = 49 г/моль
¬ реакции (8) ћэ(Al(OH)3 ) равна:
ћэ(Al(OH)2+) + Mэ(OHЦ) = fэ(Al(OH)2+) · M(Al(OH)2+) + fэ(OHЦ) · Mэ(OHЦ) = 78 г/моль
¬ реакции (9) ћэ(Al(OH)3) равна:
ћэ(AlOH2+) + Mэ(OHЦ) = fэ(Al(OH)2+) · M(AlOH2+) + fэ(OHЦ) · Mэ(OHЦ) = 39г/моль
¬ реакции (10) ћэ(Al(OH)3)равна:
ћэ(Al3+) + Mэ(OHЦ) = fэ(Al3+) · M(Al) + fэ(OHЦ) · M(OHЦ) = 26 г/моль
 ћэ(Al2(SO4)3) = fэ(Al3+) · M(Al) +fэ(SO42-) · M(SO42-) = 57 г/моль
ћэ(Al2(SO4)3) = fэ(Al3+) · M(Al) +fэ(SO42-) · M(SO42-) = 57 г/моль
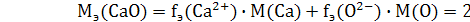 8 г/моль.
8 г/моль.
ќсновные законы химии
–аздел химии, рассматривающий массовые и объемные отношени€ между реагирующими веществами, называетс€ стехиометрией. ќснову стехиометрии составл€ют стехиометрические законы: сохранени€ массы веществ, посто€нства состава, эквивалентов, кратных отношений, объемных отношений, јвогадро. рассмотрению предложены некоторые из них.
«акон сохранени€ массы вещества
«акон сохранени€ массы вещества был сформулирован великим русским ученым ћихаилом ¬асильевичем Ћомоносовым в 1748 г. и подтвержден экспериментально им самим в 1756 г. и независимо от него французским химиком ј. Ћ. Ћавуазье в 1789 г.
¬ насто€щее врем€ он формулируетс€ так: масса веществ, вступающих в химическую реакцию, равна массе веществ, образующихс€ в результате реакции.
— точки зрени€ атомно-молекул€рного учени€ суть закона сохранени€ массы веществ заключаетс€ в том, что в химических реакци€х атомы не исчезают и не возникают из ничего, их число остаетс€ неизменным до и после реакции. ѕоэтому атомы имеют посто€нную массу и их число в результате реакции не измен€етс€, а происходит только перегруппировка атомов, то масса веществ до и после реакции остаетс€ посто€нной.
«акон сохранени€ массы €вл€етс€ частным случаем общего закона природы закона сохранени€ энергии, который утверждает, что энерги€ изолированной системы посто€нна. Ёнерги€ - это мера движени€ и взаимодействи€ различных видов материи. ѕри любых процессах в изолированной системе энерги€ не производитс€ и не уничтожаетс€, она может только переходить из одной формы в другую.
ќдной из форм энергии €вл€етс€ так называема€ энерги€ поко€, котора€ св€зана с массой уравнением Ёйнштейна:
≈ = m· C2
где E Ч энерги€ тела,
m Чмасса тела,
c Ч скорость света в вакууме, равна€ 299 792 458 м/с.
Ёто соотношение выражает эквивалентность массы и энергии. Ёквивалентность массы и энергии - физическа€ концепци€, согласно которой масса тела €вл€етс€ мерой энергии, заключЄнной в нЄм. —амое важное состоит в том, что формула Ёйнштейна раскрывает возможность взаимных превращений энергии и массы или, иначе говор€, возможность превращений энергии поко€ в другие виды энергии. —ледовательно, масса и энерги€ сохран€ютс€ не по отдельности, а вместе, что дает основание говорить об объединенном законе сохранени€ массы и энергии.
¬ химических реакци€х изменением массы, вызванным выделением или поглощением энергии, можно пренебречь. “ипичный тепловой эффект химической реакции по пор€дку величины равен 100 кƒж/моль. ѕри этом изменение массы
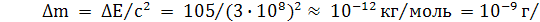 моль.
моль.
“аким образом, совершенно правомерно использование закона сохранени€ массы вещества при составлении химических уравнений и при проведении стехиометрических расчетов.
«акон посто€нства состава
—огласно закону посто€нства состава каждое химически чистое соединение всегда имеет один и тот же количественный состав независимо от способа его получени€. Ётот закон по€вилс€ в результате длительного (1801  1808) спора французских химиков ∆.ѕруста, считавшего, что отношени€ между элементами, образующими соединени€, должны быть посто€нными, и .Ѕертолле, который считал, что состав химических соединений €вл€етс€ переменным. ¬ результате тщательной экспериментальной проверки восторжествовала точка зрени€ ѕруста, считавшего состав соединений посто€нным. «акон посто€нства состава сыграл важную роль в развитии химии и до сих пор сохранил свое значение, однако вы€снилось, что не все соединени€ имеют посто€нный состав. ¬ 1912Ц1913 гг Ќ. —. урнаков установил, что существуют соединени€ переменного состава, которые он предложил назвать бертоллидами.
1808) спора французских химиков ∆.ѕруста, считавшего, что отношени€ между элементами, образующими соединени€, должны быть посто€нными, и .Ѕертолле, который считал, что состав химических соединений €вл€етс€ переменным. ¬ результате тщательной экспериментальной проверки восторжествовала точка зрени€ ѕруста, считавшего состав соединений посто€нным. «акон посто€нства состава сыграл важную роль в развитии химии и до сих пор сохранил свое значение, однако вы€снилось, что не все соединени€ имеют посто€нный состав. ¬ 1912Ц1913 гг Ќ. —. урнаков установил, что существуют соединени€ переменного состава, которые он предложил назвать бертоллидами.
—огласно современным представлени€м, посто€нство состава свойственно лишь соединени€м с молекул€рной структурой.
“аким образом, посто€нный и неизменный химический состав наблюдаетс€ только дл€ молекул (например, NH3, H2O, SO2 и т. п.), а также кристаллов с молекул€рной структурой, составл€ющих от 3 до 5 % от общего числа неорганических твердых тел. ’орошо известными примерами €вл€ютс€ твердый йод, кислород, азот, диоксид углерода, благородные газы в твердом состо€нии.
¬ насто€щее врем€ установлено, что к соединени€м переменного состава относ€тс€ не только металлические соединени€ (металлиды), но и многочисленные оксиды, сульфиды, селениды, теллуриды, нитриды, фосфиды, карбиды, силициды.
ѕрирода отклонений от стехиометрии в соединени€х переменного состава состоит в том, что при любых температурах, отличных от абсолютного нул€, в реальном кристалле существуют дефекты структуры. ѕри повышении температуры концентраци€ этих дефектов возрастает, что приводит к увеличению энтропии (неупор€доченности) системы. јбсолютно упор€доченной структурой обладает так называемый идеальный кристалл, в котором каждый атом занимает предназначенный ему узел в подрешетке. ѕри этом все узлы зан€ты, а междоузли€ вакантны. “ака€ идеализированна€ структура обладает полным пор€дком (энтропи€ равна нулю) и может быть реализована только при температуре абсолютного нул€. ѕри повышении температуры нарушени€ идеальной структуры возможны за счет возникновени€ незан€тых узлов в кристаллической решетке, по€влени€ атомов в междоузли€х или существовани€ в узлах решетки чужеродных атомов. ¬озникновение таких дефектов в реальных кристаллах приводит к нестехиометрии. ’орошо изученным соединением переменного состава €вл€етс€ сульфид железа FeS. ƒл€ природных кристаллов сульфида железа наблюдаетс€ недостаток от 10 до 20 % атомов железа против формульного состава.ƒл€ оксида титана (II) нарушение стехиометрического состава наблюдаетс€ относительно обоих сортов атомов. ¬ TiO в зависимости от условий получени€ (температура, давление кислорода) атомна€ дол€ кислорода может мен€тьс€ от 0,58 до 1,33. Ёто значит, что все составы оксида титана (II) от 0,58 до 1,00 будут характеризоватьс€ недостатком атомов кислорода (соответственно избытком атомов титана) против стехиометрии. ј составы от 1,00 до 1,33 будут иметь избыток атомов кислорода (или недостаток атомов титана) по сравнению со стехиометрическим составом.
«акон посто€нства состава был в свое врем€ сформулирован применительно к молекулам, а потому справедлив дл€ молекул€рной формы существовани€ вещества. ¬ насто€щее врем€ этот закон формулируетс€ с учетом существовани€ молекул€рной и немолекул€рной структуры вещества.
—остав молекул€рного соединени€ остаетс€ посто€нным независимо от способа его получени€. ¬ отсутствие молекул€рной структуры в данном агрегатном состо€нии состав вещества зависит от условий его получени€ и предыдущей обработки.
Ќапример, аммиак независимо от способов получени€ (пр€мой синтез из элементов, разложение аммонийных солей, действие кислот на нитриды активных металлов и т. п.) имеет посто€нный состав молекулы: на один атом азота приходитс€ три атома водорода. ј дл€ оксида титана (II) состав соединени€ зависит от условий получени€ температуры и давлени€ пара кислорода.
2.3 «акон јвогадро
»зучение свойств газов позволило италь€нскому физику ј. јвогадро в 1811г. высказать гипотезу, котора€ впоследствии была подтверждена опытными данными, и стала называтьс€ законом јвогадро: в равных объемах различных газов при одинаковых услови€х (температуре и давлении) содержитс€ одинаковое число молекул.
»з закона јвогадро вытекает важное следствие: моль любого газа при нормальных услови€х (0 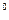 — (273 ) и давлении 101,3 кѕа ) занимает объем, равный 22,4 л. ¬ этом объеме содержитс€ 6,02× 1023 молекул газа (число јвогадро).
— (273 ) и давлении 101,3 кѕа ) занимает объем, равный 22,4 л. ¬ этом объеме содержитс€ 6,02× 1023 молекул газа (число јвогадро).
»з закона јвогадро также следует, что массы равных объемов различных газов при одинаковых температуре и давлении относ€тс€ друг к другу как мол€рные массы этих газов:
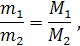
где m1 и m2 Ц массы,
 ћ1 и ћ2 Ц молекул€рные массы первого и второго газов.
ћ1 и ћ2 Ц молекул€рные массы первого и второго газов.
ѕоскольку масса вещества определ€етс€ по формуле
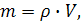
где ρЦ плотность г аза,
V Ц объем газа,
то плотности различных газов при одинаковых услови€х пропорциональны их мол€рным массам. Ќа этом следствии из закона јвогадро основан простейший метод определени€ мол€рной массы веществ, наход€щихс€ в газообразном состо€нии.
«акон јвогадро позвол€ет рассчитать плотность газа при нормальных услови€х  ) на основании отношени€ мол€рной массы ћ к объему мол€:
) на основании отношени€ мол€рной массы ћ к объему мол€:
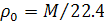 .
.
»з этого уравнени€ можно определить мол€рную массу газа:
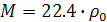 .
.
«акон объемных отношений
ѕервые количественные исследовани€ реакций между газами принадлежат французскому ученому √ей-Ћюссаку, автору известного закона о тепловом расширении газов. »змер€€ объемы газов, вступивших в реакцию и образующихс€ в результате реакций, √ей-Ћюссак пришел к обобщению, известному под названием закона простых объемных отношений: объемы вступающих в реакцию газов относ€тс€ друг к другу и объемам образующихс€ газообразных продуктов реакции как небольшие целые числа, равные их стехиометрическим коэффициентам.
Ќапример, 2H2 + O2 = 2H2O при взаимодействии двух объемов водорода и одного объема кислорода образуютс€ два объема вод€ного пара. «акон справедлив в том случае, когда измерени€ объемов проведены при одном и том же давлении и одной и той же температуре.
«акон эквивалентов
¬ведение в химию пон€тий Ђэквивалентї и Ђмол€рна€ масса эквивалентовї позволило сформулировать закон, называемый законом эквивалентов: массы (объемы) реагирующих друг с другом веществ пропорциональны мол€рным массам (объемам) их эквивалентов.
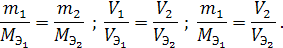
—ледует остановитьс€ на пон€тии объема мол€ эквивалентов газа. ак следует из закона јвогадро, моль любого газа при нормальных услови€х занимает объем, равный 22,4 л. —оответственно, дл€ вычислени€ объема мол€ эквивалентов газа необходимо знать число моль эквивалентов в одном моле. “ак как один моль водорода содержит 2 мол€ эквивалентов водорода, то 1 моль эквивалентов водорода занимает при нормальных услови€х объем:
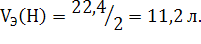
јналогичным образом можно рассчитать мол€рный объем эквивалентов кислорода:
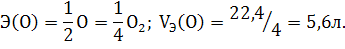
–ешение типовых задач






