При составлении уравнений ОВР нужно учесть, что число электронов, отданных восстановителем, равно числу электронов, принятых окислителем.
В химии условный заряд электрона принят за «-1» и обозначается  Для подбора стехиометрических коэффициентов можно использовать несколько методов, из которых наиболее распространены два: метод электронного баланса и метод электронно-ионных уравнений (метод полуреакций).
Для подбора стехиометрических коэффициентов можно использовать несколько методов, из которых наиболее распространены два: метод электронного баланса и метод электронно-ионных уравнений (метод полуреакций).
1. Метод электронного баланса является наиболее универсальным методом, и применим для любых окислительно-восстановительных процессов, протекающих в любых системах (растворы, расплавы, газы). В основе метода лежит принцип сравнения степеней окисления атомов в исходных веществах и в продуктах реакции с последующим составлением схемы электронного баланса.
В качестве примера рассмотрим реакцию взаимодействия дихромата калия с сероводородом в кислой среде:
 +
+ 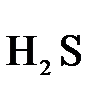 +
+  →
→ 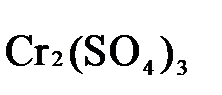 +
+  +
+  +
+ 
Для расстановки коэффициентов выполняем следующие действия.
1.1. Определяем элементы, атомы которых изменяют степень окисления:
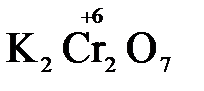 +
+  +
+  →
→  +
+  +
+  +
+ 
1.2. Находим окислитель и восстановитель в данной ОВР, составляем схему перехода электронов от восстановителя к окислителю и пишем отдельно электронные уравнения процессов окисления и восстановления, с учетом того, что количество атомов, входящих в соединение, должно сохраняться. Например, в 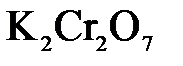 имеется два атома Cr, следовательно, в схеме они должны присутствовать:
имеется два атома Cr, следовательно, в схеме они должны присутствовать:
+5ē
-2ē
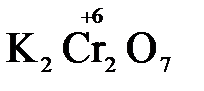 +
+  +
+  →
→  +
+  +
+  +
+ 
окислитель восстановитель
2Cr+6 + 6  = 2Cr+3 (а) - восстановление
= 2Cr+3 (а) - восстановление
S-2 - 2  = S0 (б) - окисление
= S0 (б) - окисление
1.3. Уравниваем число электронов в процессе окисления и восстановления (электронный баланс). В приведенной схеме необходимо уравнение (б) умножить на 3, тогда будет принято и отдано по 6 электронов. После умножения уравнения складываются как обычные алгебраические, а электроны сокращаются.
2Cr+6 + 6  = 2Cr+3 1
= 2Cr+3 1
+
S-2 - 2  = S0 3
= S0 3
2Cr+6 +3S-2 = 2Cr+3 + 3 S0
1.4. Полученные коэффициенты называют основными. Они переносятся в молекулярную схему реакции и ставятся перед соответствующими веществами. Так как в молекулах дихромата калия и сульфата хрома содержится по два атома хрома, двойки перед этими веществами опускаются.
K2Cr2O7 + 3H2S + H2SO4 = Cr2(SO4)3 + 3S + K2SO4 + H2O
1.5. Окончательно уравниваем число атомов каждого элемента в обеих частях молекулярного уравнения. Продукты реакции (Cr2(SO4)3, K2SO4),
имеющие коэффициенты по единице, содержат 4 моль сульфат-ионов (SO42-), которые содержатся в серной кислоте, следовательно, перед ней ставится коэффициент 4. Чтобы количество атомов водорода было одинаково в левой и правой части уравнения, перед водой ставится коэффициент 7:
K2Cr2O7 + 3H2S + 4H2SO4 = Cr2(SO4)3 + 3S + K2SO4 + 7H2O
Проверка количества остальных атомов показывает, что все коэффициенты подобраны.
2. Метод электронно-ионных уравнений (метод полуреакций) применяется для подбора коэффициентов в уравнениях реакций, протекающих в растворах. Метод оперирует с реально существующими в растворах частицами и позволяет учитывать влияние среды раствора (т.е. рН) на процессы окисления и восстановления частиц. В качестве частиц среды в водных растворах могут принимать участие следующие частицы.
Таблица 1
| Кислотность (рН) | Исходные частицы | Продукты |
| Кислая среда (рН<7) | Н+ и Н2О | Н2О и ОН- |
| Нейтральная среда (рН = 7) | Н2О | Н+ и ОН- |
| Щелочная среда (рН>7) | Н2О и ОН- | Н2О и ОН- |
В качестве примера рассмотрим ту же реакцию.
2.1. Молекулярная схема реакции:
K2Cr2O7 + H2S + H2SO4 → Cr2(SO4)3 + S + K2SO4 + H2O
2.2. Записываем это уравнение в ионно-молекулярной форме. Для этого необходимо все сильные электролиты представить в виде ионов, а слабые электролиты, газы и малорастворимые вещества оставляем в виде молекул. К сильным электролитам относятся все хорошо растворимые соли, часть кислот (HCl, HNO3, H2SO4 и др.), щелочи (LiOH, NaOH, KOH и др.). Степень окисления атомов не используют, а учитывают заряды реальных ионов и характер среды, в которой идет окислительно-восстановительный процесс.
2K+ + Cr2O7-2 + H2S + 2 H+ + SO4-2 = 2Cr+3+3SO4-2 + S0 + 2K+ +SO4-2 + H2O
кислая среда
2.3. Определяем частицы, изменившие свой заряд или состав:
Cr2O7-2 → 2Cr+3 и H2S → S0
2.4. На основании этих превращений составляем полуреакции окисления и восстановления с участием частиц среды (см. табл.1). Анион дихромата потерял 7 моль атомов кислорода, которые в кислой среде связываются 14 моль ионами водорода и превращаются в воду. Молекула сероводорода потеряла 2 моль ионов водорода.
Cr2O7-2 + 14Н+ → 2Cr+3 + 7Н2О
H2S → S0 + 2Н+
2.5. Полученные полуреакции необходимо уравнять по зарядам. В первом уравнении слева суммарный заряд равен (+12), а справа – (+6), значит, дихромат-ион присоединил 6 электронов и восстанавливается. Во втором уравнении слева (0), а справа – (+2). Молекула сероводорода потеряла 2 электрона и окислилась.
Cr2O7-2 + 14Н+ +6  → 2Cr+3 + 7Н2О
→ 2Cr+3 + 7Н2О
H2S - 2  → S0 + 2Н+
→ S0 + 2Н+
2.6. Для соблюдения электронного баланса второе уравнение необходимо умножить на 3, после чего просуммировать уравнения.
Cr2O7-2 + 14Н+ +6  → 2Cr+3 + 7Н2О 1
→ 2Cr+3 + 7Н2О 1
+
H2S - 2  → S0 + 2Н+ 3
→ S0 + 2Н+ 3
Cr2O7-2 + 14Н+ + 3H2S = 2Cr+3 + 7Н2О + 3S0 + 6Н+
После сокращения подобных частиц в левой и правой частях уравнения получим суммарное ионно-молекулярное уравнение, которое отражает смысл произошедшей реакции.
Cr2O7-2 + 8Н+ + 3H2S = 2Cr+3 + 7Н2О + 3S0
Перенос полученных коэффициентов в молекулярную схему реакции позволяет получить ее полное уравнение:
K2Cr2O7 + 3H2S + 4H2SO4 = Cr2(SO4)3 + 3S + K2SO4 + 7H2O
Иногда в реакции окислитель является одновременно и средой. Например, в реакции:
I2 + HNO3® HIO3 +NO2 +H2O
азотная кислота HNO3 одновременно содержит окислитель ион NO3- и создает кислую среду (наличие ионов Н+).
Представим молекулярное уравнение в ионно-молекулярном виде:
I2 + H+ + NO3-® IO3- +NO2 +H2O
Далее составим полуреакции окисления и восстановления с участием частиц среды (см.табл.1) и для соблюдения электронного баланса умножим первое уравнение на 10, а затем полуреакции просуммируем:
NO3- + 2H+ +ē®NO2 +H2O 10
I2 + 6H2O - 10ē ® 2IO3- + 12H+
10NO3- + 20 H+ + I2 + 6H2O®10NO2 +10 H2O + 2 IO3- + 12H+
После сокращения подобных частиц в левой и правой частях уравнения получим суммарное ионно-молекулярное уравнение:
10NO3- + 8 H+ + I2 ®10NO2 +4 H2O + 2 IO3-
Затем полученные коэффициенты перенесем в молекулярную схему реакции. Учитывая, что ионы NO3- и H+ входят в состав одного и того же соединения, а количество их разное, перед HNO3 ставится максимальный коэффициент, так как. часть азотной кислоты расходуется на создание кислой среды. Таким образом, полное уравнение:
I2 + 10HNO3®2HIO3 +10NO2 +4H2O
Метод полуреакций позволяет определить коэффициенты перед всеми веществами, участвующими в реакции, что значительно упрощает подбор дополнительных коэффициентов.






