При определенных условиях в растворе могут образоваться два или несколько анионов, которые способны давать плохо растворимые соединения. В аналитической химии необходимо определить, в каких условиях эти два аниона образуют соли, и в каком порядке эти соли осаждаются. Необходимо выяснить, какая соль будет выпадать в осадок первой, с какого момента в осадок будет выпадать вторая соль.
Пример.
 в раствор добавляют соль свинца с концентрацией ионов 0,1 моль/л
в раствор добавляют соль свинца с концентрацией ионов 0,1 моль/л

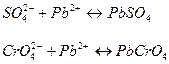
С(Рb2+) = 0,1 моль/л.
Осадок образуется тогда, когда произведение концентраций ионов в растворе становится больше, чем произведение растворимости данной соли (в крайнем случае, равно).
Поэтому в первую очередь будет выпадать в осадок хромат свинца.


Поскольку концентрация ионов свинца в хромате много меньше, чем в сульфате, то в первую очередь будет выпадать хромат свинца. Совместное осаждение двух солей произойдет тогда, когда концентрация по Рb2+ первой и второй соли сравняются между собой. 
 =
=  ;
; 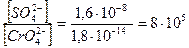

Какая соль выпадет в осадок первой и сколько процентов ее будет осаждено к началу осаждения второй соли. Оксалаты бария и кальция. Lp(BaC2O4) = 1,1×10-7; Lp(CaC2O4) = 2,3×10-9.
Ba2+ + C2O42- ® BaC2O4¯ Ca2+ + C2O42- ® CaC2O4¯
Lp(BaC2O4) = [Ba2+]×[C2O42-] Осадок образуется, если, [C2O42-] ³ Lp(BaC2O4) / [Ba2+]. Концентрация ионов бария определяется растворимостью BaC2O4
S (BaC2O4)=  .
. 
 =
=  = 4,7∙10-2;
= 4,7∙10-2;  . Поэтому сначала осаждается BaC2O4. Затем
. Поэтому сначала осаждается BaC2O4. Затем 
 происходит совместное осаждение.
происходит совместное осаждение.
Lp(BaC2O4) / [Ba2+] = Lp(CaC2O4) / [Ca2+]. Lp(CaC2O4) / Lp(BaC2O4) = [Ca2+ ]/ [Ba2+]=0,25. если концентрация [Ba2+] = 1моль, то 0,25 моль еще не выпадет в осадок. 75 % соли будет осаждено.
Плохо растворимое основание выпадает в осадок при определенной величине рН, которая зависит от концентрации катиона, образующего это основание. Эта величина рН называется рН гидратообразования. Осаждение называют полным, когда концентрация в растворе сама стоновится раной 10-6 моль/л.
Пример. Рассчитать рН гидратообразования при концентрации ионов Mg 10-2 моль/л и при полном осаждении гидроксида из раствора.
[Mg2+] = 10-2 моль/л
C (Mg(OH)2) = 10-6 моль/л. Mg(OH)2 «Mg2+ + 2OH-
Lp(Mg(OH)2) = [Mg2+ ]×[OH-]2 = 8∙10-8 моль/л
[OH-] = (Lp(Mg(OH)2) / [Mg2+ ])1/2
[OH-] = (8∙10-8 моль/л / 10-2)1/2=2,45∙10-3  pOH = 2,61 pH=14-2,61=11,4
pOH = 2,61 pH=14-2,61=11,4
[OH-] = (8∙10-8 моль/л / 10-6)1/2=2,8∙10-7 
ВЗАИМНОЕ РАСТВОРЕНИЕ ПЛОХО РАСТВОРИМЫХ ОСАДКОВ
При анализе иногда приходится переводит одно плохо растворимое соединение в другое, которое растворяется еще хуже, или в соль с большим произведением растворимости для того, чтобы в дальнейшем вновь образовавшуюся соль можно было растворить и вновь провести анализ.
Перевод соли из лучше растворимой в более труднорастворимую
 CaSO4 + CO32- ® CaCO3¯ + SO42-
CaSO4 + CO32- ® CaCO3¯ + SO42-
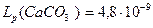
Равновесная концентрация в первой соли 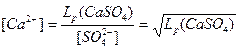 =
= 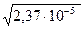 =4,8∙10-3
=4,8∙10-3
Равновесная концентрация во второй соли  =
=  =6,9∙10-5.
=6,9∙10-5.
Произведение растворимости [Ca2+][CO32-] будет увеличиваться за счет концентрации Ca2+ . СaSO4 будет подавать все новые и новые катионы кальция из осадка, а они будут связываться с ионом CО32-. Так будет происходить до тех пор, пока полностью не растворится СaSO4 или не будет исчерпан запас ионов CО32-.
Перевод соли из труднорастворимой в лучше растворимую
BaSO4 + Na2CO3 «Ba CO3 + Na2 SO4

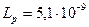
Если концентрация ионов CO32- большая, а SO42- мала, тогда можно управлять соотношением концентрации ионов. BaSO4 начнет переходить в Ba CO3 когда начнет выполняться неравенство [Ba2+]1 > [Ba2+]2
[Ba2+]1=  [Ba2+]2=
[Ba2+]2= 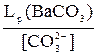
 >
> 
 >
>  =46,4 В этом случае начнется осаждение и равновесие сместится вправо.
=46,4 В этом случае начнется осаждение и равновесие сместится вправо.






