| № опыта | Формулы кислоты и щелочи | Масса кислоты m к , г | Масса щелочи m щ , г | Масса стакана m ст , г | Изменение температуры в опыте, °С | Концентрация кислоты С Э, моль/дм3 | Теплота нейтрализации Q нейтр , кДж/моль | D Н экспнейтр , кДж/моль | D Н теорнейтр , кДж/моль | Относительная погрешность П, % | |
| t нач | t кон | ||||||||||
| 1 | |||||||||||
| 2 | |||||||||||
Обработка экспериментальных данных
Часть 1. Определение изменения энтальпии растворения безводной соли
1. Изменение температуры в калориметрическом опыте вычислить по формуле
D t = t кон – t нач. (9)
2. Суммарную теплоемкость системы оценить по уравнению
Cw = [(m воды + m соли) · С воды + m стакана · С стекла], Дж/К,
где m воды , m соли , m стакана – масса воды, соли и стакана, соответственно, г; С воды , С стекла – удельные теплоемкости воды и стекла, равные,соответственно: С воды = 4,184Дж/г · К; С стекла = = 0,753 Дж/г · К.
3. Тепловой эффект растворения 1 моль соли рассчитать по формуле
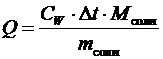 , Дж/моль,
, Дж/моль,
где М соли – молярная масса безводной соли, взятой для опыта, г/моль.
4. Записать величину изменения энтальпии растворения соли, исходя из уравнения (8):
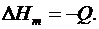
5. По данным параллельных опытов вычислить среднее значение D 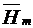 .
.
6. Выбрать теоретическое значение изменения энтальпии растворения данной соли (см. прил. 3) в зависимости от моляльной концентрации полученного раствора соли, которую оценивают по формуле
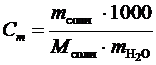 , моль/кг.
, моль/кг.
7. Рассчитать относительную погрешность опыта в процентах:
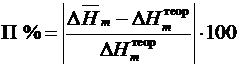 %.
%.
6. Экспериментальные данные и результаты расчетов занести в таблицу формы 4.
Форма 4
Экспериментальные и расчетные данные для процесса растворения соли (указать название соли)
| № опыта | М соли , г/моль | D t,°C | C w, Дж/К | Q, кДж/моль | 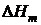 , кДж/моль , кДж/моль
| 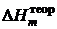 , кДж/моль , кДж/моль
| П, % |
| 1 2 |
Часть 2. Определение изменения энтальпии реакции нейтрализации сильной кислоты сильным основанием
1. Вычислить изменение температуры в процессе нейтрализации D t по формуле (9).
2. Рассчитать количество теплоты, выделившееся в результате опыта:
Q = [(m к + m щ) · С воды + m ст · С стекла] × D t, Дж,
где m к , m щ , m ст – массы кислоты, щелочи и стакана, соответственно, г. Плотности растворов кислоты и щелочи принять равными 1 г/см3.
3. Вычислить тепловой эффект реакции нейтрализации:
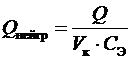 , Дж/моль,
, Дж/моль,
где V к – объем кислоты, дм3; С Э – молярная эквивалентная концентрация раствора кислоты,моль/дм3 (н).
4. Записать изменение энтальпиинейтрализации:
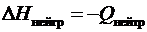 .
.
5. По даннымпараллельных опытов вычислить среднее значение 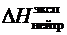 .
.
6. По данным прил. 4 выбрать теоретические значения изменения энтальпии нейтрализации в зависимости от температуры и определить относительную погрешность опыта в процентах:
 %.
%.
7. Результаты опытов и расчетные данные занести в таблицу формы 3.
8. Оформить метрологическую карту средств измерения и сформулировать выводы.
Контрольные задания
Вариант 1
1. Исходя из уравнения реакции, вычислить энтальпию образования метилового спирта 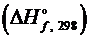 .
.
СН3ОН(ж) + 3/2О2(г) = СО2(г) + 2Н2О(ж); 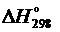 = –726,5 кДж.
= –726,5 кДж.
2. Пользуясь справочными данными, показать, что в стандартных условиях реакция
Cu(тв) + ZnO(тв) = CuO(тв) + Zn(тв)
невозможна, если 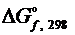 для ZnO и CuO равны, соответственно, -318,19 и -127,19 кДж/моль.
для ZnO и CuO равны, соответственно, -318,19 и -127,19 кДж/моль.
3. В каком направлении протекает реакция в изолированной системе
С (графит) + СО2(г) ↔ 2СО(г)?
4. По следующим термохимическим уравнениям установить, являются ли эти реакции экзо- или эндотермическими?
а) CS2(ж) + 3O2(г) = CO2(г) + 2SO2(г)  = -1075 кДж;
= -1075 кДж;
б) Fe2O3 + 3Н2 = 2Fe(к) + 3Н2О(г)  = 96,61 кДж.
= 96,61 кДж.
Вариант 2
1. Восстановление оксида железа (III) водородом протекает по уравнению
Fe2O3(к) + 3H2(г) = 2Fe(к) + 3H2O(г) ,  = 96,61 кДж.
= 96,61 кДж.
Возможна ли данная реакция при стандартных условиях, если 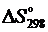 = 38,7 Дж/(моль · К)? При какой температуре начнется восстановление оксида железа (III)?
= 38,7 Дж/(моль · К)? При какой температуре начнется восстановление оксида железа (III)?
2. Определить стандартную теплоту образования сероуглерода по тепловому эффекту реакции
CS2(ж) + 3O2(г) = CO2(г) + 2SO2(г),  = -1075 кДж.
= -1075 кДж.
3. Восстановление Fe3O4 оксидом углерода идет по уравнению
Fe3O4(к) + СО(г) = 3FeO(к) + СО2(г).
Чему равно 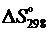 этой реакции?
этой реакции?
4. Какие реакции являются экзотермическими?
а) Fe2O3(к) + 3H2(г) = 2Fe(к) + 3H2O(г),  = 96,61 кДж;
= 96,61 кДж;
б) MgO(тв) + Al2O3(тв) = (MgAl2)O4(тв),  = -39 кДж.
= -39 кДж.
Вариант 3
1. Написать термохимическое уравнение реакции между СО(г) и водородом, в результате которой образуются СН4(г) и Н2О(г). Сколько теплоты выделится при протекании этой реакции?
2. По следующим термохимическим уравнениям установить, являются ли данные реакции экзо- или эндотермическими?
а) 2Al2O3(тв) + 6SO2(г) + 3O2(г) = 2Al2(SO4)3(тв),  = -1750 кДж;
= -1750 кДж;
б) РCl5(г) = РCl3(г) + Cl2(г),  = -88 кДж.
= -88 кДж.
3. Рассчитать стандартную энтропию оксида железа (III), если известна энтропия реакции
4FeО(к) + О2(г) = 2Fe2O3(к), 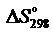 = -259 Дж/К.
= -259 Дж/К.
4. Рассчитать изменение энергии Гиббса процесса алюмотермического восстановления оксида железа при температурах 298 и 500 К.
Fe2O3(к) + 3Аl (к) = 2Fe(к) + Аl 2O3(к).
Как температура влияет на протекание реакции в прямом направлении?
Вариант 4
1. При взаимодействии газообразного сероводорода и диоксида углерода образуются пары воды и сероуглерод CS2(г). Написать термохимическое уравнение этой реакции и вычислить её тепловой эффект.
2. По термохимическому уравнению установить, являются ли реакции экзо- или эндотермическими?
N2H4(г) + O2(г) = N2(г) + 2H2O(г),  = 579 кДж.
= 579 кДж.
3. Определить стандартную энергию Гиббса реакции
СН4 + 2Н2О(г) = СО2 + 4Н2.
Возможно ли самопроизвольное течение реакции в прямом направлении?
4. Вычислить изменение внутренней энергии при испарении 100 г воды при температуре 20 °С, если на испарение 1 моль воды расходуется 40,7 кДж тепла. Пар следует считать идеальным газом, а объемом жидкой воды можно пренебречь.
Вариант 5
1. Прямая или обратная реакция будет протекать в изолированной системе
СН4(г) + СО2(г) ↔ 2СО2(г) + 2Н2(г)?
2. Газообразный этиловый спирт можно получить при взаимодействии этилена С2Н4(г) и водяных паров. Написать термохимическое уравнение этой реакции, вычислить её тепловой эффект при стандартных условиях.
3. Возможно ли при температурах 298 и 1000 К восстановление оксида железа (III) до свободного металла по уравнению
Fe2O3(к) + 3Н2(г) = 2Fe(к) + 3Н2О(г)?
4. Вычислить изменение внутренней энергии при испарении 90 г воды при 100 °С, если на испарение 1 моль воды расходуется 40,7 кДж тепла. Пар следует считать идеальным газом, а объемом жидкой воды можно пренебречь.
Вариант 6
1. Вычислить тепловой эффект реакции
H2S(г) + 1,5O2(г) = Н2О(ж) + SO2(г).
2. Прямая или обратная реакция будет протекать при стандартных условиях в системе
4НCl(г) + О2(г) = 2Cl2(г) + 2Н2О(г)?
3. Рассчитать изменение внутренней энергии реакции при стандартных условиях:
Na2CO3(к) = Na2O(к) + CO2(г).
Какое количество теплоты требуется на разложение 10 кг карбоната натрия?
4. Какой знак будет иметь 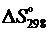 для реакции
для реакции
2Н2О(г) + С(графит) = 2Н2(г) + СО2(г)?
Сопоставить вывод с результатами расчетов.
Вариант 7
1. Прямая или обратная реакция будет протекать при стандартных условиях в системе
N2O(г) + NO(г) ↔ NO2(г) + N2(г)?
2. Вычислить теплоту образования карбида кальция, исходя из теплового эффекта реакции
СаО(к) + 3С(к) = СаС2(к) + СО(г);  = 480 кДж/моль.
= 480 кДж/моль.
3. Рассчитать стандартную энтропию реакции образования оксида железа (III) из простых веществ по реакции
2Fe(к) + 3О2(г) = 2Fe2O3(к),
используя следующие данные:
2Fe(к) + О2(г) = 2FeO(к); 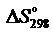 = -145 Дж/К;
= -145 Дж/К;
4FeO(к) + О2(г) = 2Fe2O3(к); 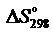 = -259 Дж/К.
= -259 Дж/К.
4. По следующим термохимическим уравнениям установить, являются ли эти реакции экзо- или эндотермическими?
а) CS2(ж) + 3O2(г) = CO2(г) + 2SO2(г);  = -1075 кДж;
= -1075 кДж;
б) Na2CO3 + SiO2 = Na2SiO3 + CO2;  = 819,29 кДж.
= 819,29 кДж.
Вариант 8
1. Прямая или обратная реакция протекает при стандартных условиях в системе
2N2O(г) + O2(г) ↔ 4NO(г)?
2. При взаимодействии 3 моль оксида азота N2O с аммиаком образуются азот и пары воды. Тепловой эффект реакции равен
-877,76 кДж. Написать термохимическое уравнение этой реакции.
3. По следующим термохимическим уравнениям установить, являются ли реакции экзо- или эндотермическими?
а) Zr(тв) + ZrCl4(г) = 2ZrCl2(г);  = -215 кДж;
= -215 кДж;
б) Zr(тв) + 2Cl2(г) = ZrCl4(г);  = -867 кДж.
= -867 кДж.
4. Прямая или обратная реакция будет протекать при стандартных условиях в изолированной системе
С2Н2(г) + 5/2О2(г) ↔ 2СО(г) + Н2О(ж)?
Вариант 9
1. При сгорании газообразного аммиака образуются пары воды и оксида азота NO(г). Написать термохимическое уравнение этой реакции, вычислив её тепловой эффект в расчете на 1 моль аммиака.
2. По следующим термохимическим уравнениям установить, являются ли реакции экзо- или эндотермическими?
а) 2As(тв) + 3F2(г) = 2AsF3(г),  = -184,2 кДж;
= -184,2 кДж;
б) AsF5(г) = AsF3(г) + F2(г),  = -317 кДж.
= -317 кДж.
3. Определить 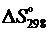 для реакции
для реакции
Fe2O3 + 3Н2 = 2Fe(к) + 3Н2О(г).
4. В каком направлении протекает реакция
Al2O3 + 3SO2 ↔ Al2(SO4)3
при стандартных условиях?
Вариант 10
1. Тепловой эффект реакции сгорания 1 моль жидкого бензола с образованием паров воды и диоксида углерода равен
-3135,58 кДж. Составить термохимическое уравнение этой реакции и вычислить теплоту образования жидкого бензола.
2. По следующим термохимическим уравнениям установить, являются ли реакции экзо- или эндотермическими.
а) 4Аs(тв) + 3O2(г) = 2As2O3(тв),  = -1328 кДж;
= -1328 кДж;
б) 2PbO2(тв) = 2PbO(тв) + O2(г),  = -116 кДж.
= -116 кДж.
3. Реакция горения ацетилена идет по уравнению
С2Н2(г) + 5/2О2(г) = 2СО2(г) + Н2О(ж).
Вычислить 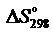 и
и 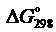 реакции.
реакции.
4. Написать термохимическое уравнение реакции между СО(г) и водородом, в результате которой образуются СН4(г) и Н2О(г). Сколько теплоты выделится при протекании реакции при V = = const?
Вариант 11
1. Вычислить 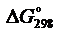 реакции и определить направление протекания процесса
реакции и определить направление протекания процесса
NH3(г) + HCl(г) ↔ NH4Cl(к).
2. Рассчитать стандартный тепловой эффект реакции
Fe2O3(к) + 3Н2(г) = 2Fe(к) + 3Н2О(г).
3. Вычислить значение 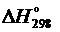 для протекающих в организме реакций превращения глюкозы:
для протекающих в организме реакций превращения глюкозы:
а) С6Н12О6(к) + 6О2(г) = 6СО2(г) + 6Н2О(ж);
б) С6Н12О6(к) = 2С2Н5ОН(ж) + 2СО2(г).
Какая реакция поставляет организму больше тепла?
4. Прямая или обратная реакция будет протекать при стандартных условиях в изолированной системе
2NO(г) + О2(г) ↔ 2NO2(г)?
Вариант 12
1. Реакция горения метилового спирта выражается термохимическим уравнением
СН3ОН(ж) + 3/2О2(г) = СО2(г) + 2Н2О(ж).
Вычислить тепловой эффект этой реакции, если известно, что мольная теплота парообразования СН3ОН(ж) равна 37,4 кДж.
2. По следующим термохимическим уравнениям установить, являются ли реакции экзо- или эндотермическими?
а) С(т) + Н2О(г) = СО(г) + Н2(г), 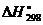 = 132 кДж;
= 132 кДж;
б) СuO(тв) + C(тв) = Cu(тв) + CO(г), 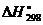 = -46 кДж.
= -46 кДж.
3. Реакция горения бензола выражается уравнением
С6Н6(ж) + 7,5О2(г) = 6СО2(г) + 3Н2О(г).
Вычислить изменение энтропии реакции.
4. Газообразный этиловый спирт можно получить при взаимодействии этилена и водяных паров. Вычислить изменение энергии Гиббса реакции.
Вариант 13
1. Определить тепловой эффект реакции
NaH(кр) + H2O(ж) = NaOH(ж) + H2(г) ,
если 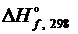 NaH и NaOH равны, соответственно, -56,94 и 469,47 кДж/моль.
NaH и NaOH равны, соответственно, -56,94 и 469,47 кДж/моль.
2. В каком направлении протекает реакция в изолированной системе реакции
Н2(г) + 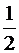 О2(г) ↔ Н2О(г)?
О2(г) ↔ Н2О(г)?
Определить не производя расчетов.
3. Реакция горения этилового спирта выражается термохимическим уравнением
С2Н5ОН(ж) + 3О2(г) = 2СО2(г) + 3Н2О(ж).
Вычислить тепловой эффект реакции, если известно, что мольная теплота парообразования этилового спирта 42,36 кДж/моль.
4. У каких реакций при повышении давления равновесие сдвинется в прямом направлении?
а) MgO(тв) + Al2O3(тв) ↔ (MgAl2)O4(тв);
б) СН3ОН(ж) + 3/2О2(г) ↔ СО2(г) + 2Н2О(ж).
Вариант 14
1. Реакция горения аммиака выражается термохимическим уравнением
4NH3(г) + 3O2(г) = 2N2(г) + 6H2O(ж),  = -1530,28 кДж.
= -1530,28 кДж.
Вычислить теплоту образования аммиака.
2. Какие реакции являются экзотермическими?
а) С(тв) + Н2О(г) = СО(г) + Н2(г),  = +132 кДж;
= +132 кДж;
б) СuO(тв) + C(тв) = Cu(тв) + CO(г),  = -46 кДж;
= -46 кДж;
в) MgO(тв) + Al2O3(тв) = (MgAl2)O4(тв) ,  = -39 кДж.
= -39 кДж.
3. В каком направлении протекает реакция в изолированной системе реакции
Н2(г) + О2(г) ↔ 2Н2О2(ж)?
Определить не производя расчетов.
4. В каком направлении сместится равновесие при повышении температуры?
FeO(тв) + CO(г) ↔ Fe(тв) + CO2(г),  = -17 кДж.
= -17 кДж.
Вариант 15
1. Определить тепловой эффект реакции
2PbS + 3О2 = 2PbO + 2SO2,
если 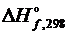 РbS и PbO равны, соответственно, -94,28 и
РbS и PbO равны, соответственно, -94,28 и
-217,86 кДж/моль.
2. В каком направлении протекает реакция в изолированной системе реакции
Na2CO3 + SiO2 ↔ Na2SiO3 + CO2
при стандартных условиях?
Определить не производя расчетов.
3. В каком направлении сместится равновесие при повышении температуры?
Na2O + SiO2 ↔ Na2SiO3,  = -243,5 кДж.
= -243,5 кДж.
4. Определить стандартную энтальпию образования РН3, исходя из уравнения
2РН3(г) + 4О2(г) = Р2О5(к) + 3Н2О(ж), 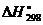 = -2360 кДж,
= -2360 кДж,
если 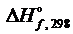 (Р2О5) = -1549 кДж.
(Р2О5) = -1549 кДж.
Работа 4






