| ион | Br- | CH3COO- | CO 
| Cl- | F- | I- | NO 
| PO 
| S2- | SO 
|
| Ag+ | н | м | н | н | р* | н | р | н | н | м |
| Al3+ | *р | + | – | *р | м | *р | *р | н | + | *р |
| Ba2+ | р | р* | н | р | м | р | р | н | р* | н |
| Be2+ | *р | + | [н] | *р | *р* | *р | *р | н | + | *р |
| Ca2+ | р | р* | н | р | н | р | р | н | р* | м |
| Cd2+ | *р | *р* | [н] | *р | *р* | *р | *р | н | н | *р |
| Co2+ | *р | *р* | [н] | *р | *р* | *р | *р | н | н | *р |
| Cr3+ | *р | + | – | *р | м | н | *р | н | [н] | *р |
| Cu2+ | *р | *р* | [н] | *р | *р* | – | *р | н | н | *р |
| Fe2+ | *р | *р* | [н] | *р | м | *р | *р | н | н | *р |
| Fe3+ | *р | – | – | *р | н | – | *р | н | – | *р |
| Hg2+ | м | *р* | – | *р | + | н | + | н | н | + |
Hg 
| н | м | н | н | м | н | + | н | – | н |
| K+ | р | р* | р* | р | р* | р | р | р* | р* | р |
| Li+ | р | р* | р* | р | н | р | р | м | р* | р |
| Mg2+ | *р | *р* | м | *р | м | *р | *р | н | н | *р |
| Mn2+ | *р | *р* | [н] | *р | *р* | *р | н | н | н | *р |
NH 
| *р | *р* | *р* | *р | *р* | *р | *р | – | + | *р |
| Na+ | р | р* | р* | р | р* | р | р | р* | р* | р |
| Ni2+ | *р | *р* | [н] | *р | *р* | *р | *р | н | н | *р |
| Pb2+ | м | *р* | [н] | м | м | м | *р | н | н | н |
| Sn2+ | + | + | – | + | *р* | м | + | н | н | *р |
| Sr2+ | р | р* | н | р | н | р | р | н | р* | р |
| Tl2+ | м | р* | м | м | р* | н | р | н | н | м |
| Zn2+ | *р | *р* | [н] | р | м | *р | *р | н | н | *р |
Обозначения: р – хорошо растворимая соль (>0,1 моль/л)
м – малорастворимая соль (0,1 – 0,001 моль/л)
н – практически нерастворимая соль (<0,001 моль/л)
[н] – не осаждается из раствора вследствие необратимого гидролиза
+ - полностью реагирует с водой
– - не существует (соль не получена)
*р – гидролиз по катиону
р* - гидролиз по аниону
Стандартные электродные потенциалы в водных растворах
| Электрод | Е 0, В | Электрод | Е 0, В |
| Li+/Li | – 3,045 | Tl +/Tl | – 0,336 |
| Rb+/Rb | – 2,925 | Co2+/Co | – 0,277 |
| K+/K | – 2,924 | Ni2+/Ni | – 0,257 |
| Cs+/Cs | – 2,923 | Mo3+/Mo | – 0,200 |
| Ba2+/Ba | – 2,912 | Sn2+/Sn | – 0,136 |
| Fr+/Fr | – 2,900 | Pb2+/Pb | – 0,126 |
| Sr2+/Sr | – 2,868 | Fe3+/Fe | – 0,036 |
| Ra2+/Ra | – 2,800 | 2H+/H2 | 0,000 |
| Ca2+/Ca | – 2,866 | Ge4+/Ge | +0,124 |
| La3+/La | – 2,379 | Ge2+/Ge | +0,240 |
| Y3+/Y | – 2,372 | Re3+/Re | +0,300 |
| Na+/Na | – 2,714 | Bi3+/Bi | + 0,308 |
| Mg2+/Mg | – 2,363 | Cu2+/Cu | + 0,342 |
| Be2+/Be | – 1,847 | Tc2+/Tc | +0,400 |
| Al3+/Al | – 1,662 | Ru2+/Ru | +0,455 |
| Ti 2+/Ti | – 1,628 | Cu+/Cu | + 0,521 |
| Zr4+/Zr | – 1,580 | Rh2+/Rh | +0,600 |
| Hf4+/Hf | – 1,550 | Tl 3+/Tl | + 0,741 |
| Ti 3+/Ti | – 1,370 | Rh3+/Rh | +0,758 |
| V2+/ V | – 1,186 | Po4+/Po | +0,760 |
| Mn 2+/ Mn | – 1,180 | Hg  /Hg /Hg
| +0,797 |
| Cr 2+/Cr | – 0,913 | Ag+/Ag | + 0,799 |
| Zn2+/Zn | – 0,763 | Hg 2+/Hg | + 0,851 |
| Cr3+/Cr | – 0,744 | Pd2+/Pd | +0,951 |
| Ta 3+/Ta | – 0,600 | Ir3+/Ir | +1,156 |
| Ga3+/Ga | – 0,549 | Pt2+/Pt | + 1,190 |
| Fe2+/Fe | – 0,440 | Au3+/Au | + 1,498 |
| Cd2+/Cd | – 0,403 | Au+/Au | + 1,691 |
| In3+/In | – 0,338 |
Стандартные окислительно-восстановительные потенциалы
| Элемент | Полуреакция | Е 0, В |
| Ag | Ag+ + 1 ē = Ag | +0,80 |
| AgCl + 1 ē = Ag + Cl- | + 0,22 | |
| Ag2+ + 1 ē = Ag+ | + 1,98 | |
| Ag2О + Н2О + 2 ē = 2Ag + 2ОН- | + 0,34 | |
| 2AgО + Н2О + 2 ē = Ag2О + 2ОН- | + 0,607 | |
| Ag2S + 2 ē = 2Ag + S2- | – 0,691 | |
| Ag2S + 2Н+ + 2 ē = 2Ag + Н2S | – 0,0366 | |
| AgI + 1 ē = Ag + I- | – 0,15 | |
| Al | AlO  + 2H2O + 3 ē = Al + 4OH- + 2H2O + 3 ē = Al + 4OH-
| – 2,35 |
| Al(OH)3 + 3 ē = Al + 4OH- | – 2,328 | |
| As | H3AsO4 + 2H+ + 2 ē = HAsO2 + 2H2O | + 0,56 |
AsO  + 2H2O + 2 ē = AsO + 2H2O + 2 ē = AsO  + 4OH- + 4OH-
| – 0,71 | |
| Be | Be2O  + 3H2O + 4 ē = 2Be + 6OH- + 3H2O + 4 ē = 2Be + 6OH-
| – 2,63 |
| Bi | NaBiO3 + 6H+ + 2 ē = Bi3+ + 3H2O + Na+ | +1,81 |
| Br | BrO3- + 3H2O + 6 ē = Br-+ 6OH- | +0,61 |
| Br2 + 2 ē = 2Br- | +1,09 | |
| HBrO + H+ + 2 ē = Br- + H2O | +1,34 | |
| Cl | Cl2 + 2 ē = 2Cl- | +1,36 |
| ClO3- + 6H+ + 6 ē = Cl- + 3H2O | +1,46 | |
| ClO- + H2O + 2 ē = Cl- + 2OH- | +0,92 | |
| 2ClO- + 2H2O + 2 ē = Cl2 + 4OH- | +1,56 | |
ClO  + 2H2O + 4 ē = Cl- + 4OH- + 2H2O + 4 ē = Cl- + 4OH-
| +0,76 | |
ClO  + H2O + 2 ē = ClO- + 2OH- + H2O + 2 ē = ClO- + 2OH-
| +0,66 | |
ClO 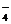 + 8H+ + 8 ē = Cl- + 4H2O + 8H+ + 8 ē = Cl- + 4H2O
| +1,389 | |
ClO 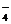 + H2O + 2 ē = ClO + H2O + 2 ē = ClO  + 2OH- + 2OH-
| +0,36 | |
| HClO2 + 2H+ + 2 ē = HClO + H2O | + 1,645 | |
| Cr | Cr2O72- + 14H+ + 6 ē = 2Cr3+ + 7H2O | +1,33 |
CrO  +2H2O + 3 ē = CrO2- + 4OH- +2H2O + 3 ē = CrO2- + 4OH-
| - 0,21 | |
CrO  + 4H2O + 3 ē = Cr3+ + 8OH- + 4H2O + 3 ē = Cr3+ + 8OH-
| +1,45 | |
CrO  + 8H+ + 3 ē = Cr3+ + 4H2O + 8H+ + 3 ē = Cr3+ + 4H2O
| +1,47 | |
| Cr(OH)3 + 3 ē = Cr + 3OH- | – 1,48 | |
| CrO3 + 6H+ + 3 ē = Cr3+ + 3H2O | – 0,11 | |
CrO  + 4H2O + 3 ē = Cr(OH)3 + 5OH- + 4H2O + 3 ē = Cr(OH)3 + 5OH-
| – 0,13 | |
| H | 2H2O + 2 ē = H2 + 2OH- | – 0,83 |
| H2O2 + 2H+ + 2 ē = 2H2O | +1,776 | |
| I | 2IO3- + 12H+ + 10 ē = I2 + 6H2O | +1,195 |
| 2IO- + 4H+ + 2 ē = I2 + 2H2O | +1,10 | |
| HIO + H+ + 2 ē = I- + H2O | +0,987 | |
| IO- + H2O + 2 ē = I- + 2OH- | +0,485 | |
IO  + 6H+ + 6 ē = I- + 3H2O + 6H+ + 6 ē = I- + 3H2O
| +1,085 | |
IO  + 2H2O+ 4 ē = IO- + 4OH- + 2H2O+ 4 ē = IO- + 4OH-
| +0,15 | |
IO  + 3H2O+ 6 ē = IO- + 6OH- + 3H2O+ 6 ē = IO- + 6OH-
| +0,26 | |
| I2 + 2 ē = 2I- | +0,54 | |
| 2HIO + 2H+ + 2 ē = I2 + 2H2O | +1,439 | |
| Fe | Fe(OH)3 + 1 ē = Fe(OH)2 + OH- | – 0,56 |
| Fe2O3 + 4H+ + 2 ē = 2FeOH+ + H2O | +0,16 | |
| Mn | MnO2 + 4H+ + 2 ē = Mn2+ + 2H2O | +1,24 |
MnO 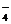 + 8H+ + 5 ē = Mn2+ + 4H2O + 8H+ + 5 ē = Mn2+ + 4H2O
| +1,53 | |
MnO 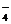 + 1 ē = MnO42- + 1 ē = MnO42-
| +0,56 | |
| MnO2 + 4OH- + 2 ē = Mn2+ + 2H2O | +1,15 | |
MnO 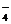 + 2H2O + 2 ē = MnO2 + 4OH- + 2H2O + 2 ē = MnO2 + 4OH-
| +0,60 | |
MnO 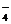 + 4H+ + 3 ē = MnO2 + 2H2O + 4H+ + 3 ē = MnO2 + 2H2O
| +1,679 | |
MnO 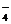 + 2H2O + 3 ē = MnO2 + 4OH- + 2H2O + 3 ē = MnO2 + 4OH-
| +0,595 | |
| Mn(OH)2 + 2 ē = Mn + 2OH- | – 1,56 | |
| Mn(OH)3 + 1 ē = Mn(OH)2 + OH- | +0,15 | |
| Mn2O3 + 6H+ + 2 ē = 2Mn2+ + 3H2O | +1,485 | |
| N | NO  + 2H+ + 1 ē = NO2 + H2O + 2H+ + 1 ē = NO2 + H2O
| +0,77 |
| N2 + 2H2O + 6H+ + 6 ē = 2NH4OH | 0,092 | |
| 3N2 + 2H+ + 2 ē = 2HN3 | – 3,09 | |
N2H  + 3H+ + 2 ē = 2NH + 3H+ + 2 ē = 2NH 
| +1,275 | |
| N2O + 2H+ + 2 ē = N2 + H2O | +1,766 | |
N2O4 + 2 ē = 2NO 
| +0,867 | |
| N2O4 + 2H+ + 2 ē = 2HNO2 | +1,065 | |
| N2O4 + 4H+ + 4 ē = 2NO + 2H2O | +1,035 | |
| 2NO + 2H+ + 2 ē = N2O + H2O | +1,591 | |
| 2NO + H2O + 2 ē = N2O + 2OH- | +0,76 | |
| HNO2 + H+ + 1 ē = NO + H2O | +0,983 | |
NO  + 3H+ + 2 ē = HNO2 + H2O + 3H+ + 2 ē = HNO2 + H2O
| +0,94 | |
NO  + 2H+ + 2 ē = NO2- + H2O + 2H+ + 2 ē = NO2- + H2O
| +0,84 | |
NO  + H2O + ē = NO + 2OH- + H2O + ē = NO + 2OH-
| – 0,46 | |
NO  + 4H+ + 3 ē = NO + 2H2O + 4H+ + 3 ē = NO + 2H2O
| +0,96 | |
2NO  + 2H2O + 4 ē = N2O + 2H2O + 4 ē = N2O  + 4OH- + 4OH-
| – 0,18 | |
2NO  + 3H2O + 4 ē = N2O + 6OH- + 3H2O + 4 ē = N2O + 6OH-
| +0,15 | |
2NO  + 2H2O + 2 ē = N2O4 + 4OH- + 2H2O + 2 ē = N2O4 + 4OH-
| – 0,85 | |
2NO  + 4H+ + 2 ē = N2O4 + 2H2O + 4H+ + 2 ē = N2O4 + 2H2O
| +0,803 | |
NO  + H2O + 2 ē = NO + H2O + 2 ē = NO  + 2OH- + 2OH-
| +0,01 | |
| О
| O2 + 2H+ + 4 ē = 4OH- | +0,40 |
| O2 + 2H+ + 2 ē = H2O2 | +0,69 | |
| O2 + 4H+ + 4 ē = 2H2O | +1,229 | |
| O2 + 2H2O + 2 ē = H2O2 + 2OH- | – 0,146 | |
| O3 + 2H+ + 2 ē = H2O + O2 | +2,076 | |
| O2 + 2H2O + 4 ē = 4OH- | +0,40 | |
| O3 + 2H2O + 2 ē = O2 + 2OH- | +1,24 | |
| P | H3PO4 + 5H+ + 5 ē = P + 4H2O | -0,41 |
| H3PO4 + 2H+ + 2 ē = H3PO3 + H2O | -0,28 | |
| Pb | [Pb(OH)4]2- + 2 ē = Pb + 4OH- | -0,52 |
| PbO2 + 4H+ + 2 ē = Pb2+ + 2H2O | +1,46 | |
| PbO2 + 2 ē = PbO22- | +1,70 | |
| S | S + 2 ē = S2- | -0,48 |
| S + H2О + 2 ē = HS- + ОН- | - 0,478 | |
| SO42- + 10H+ + 8 ē = H2S + 4H2O | +0,30 | |
| S + 2H+ + 2 ē = H2S | +0,142 | |
| SO2 + 4H+ + 4 ē = S + 2H2O | +0,45 | |
| SO42-+ 4H+ + 2 ē = SO2 + 2H2O | +0,16 | |
| SO42- + 8H+ + 6 ē = S + 4H2O | +0,35 | |
| SO42- + 8H+ + Cu2+ + 8 ē = CuS + 4H2O | +0,42 | |
| SO42- + 2H+ + 2 ē = SO32- + H2O | -0,10 | |
| Se | SeO42- + 2H+ + 2 ē = SeO32- + H2O | +1,15 |






