Краткие теоретические сведения
Ионным произведением воды K В называют произведение активности (или концентрации) ионов водорода на активность (или концентрацию) гидроксид-ионов
K В = а  × а
× а  = [Н +]×[ОН – ]. (38)
= [Н +]×[ОН – ]. (38)
K В характеризует равновесие процесса диссоциации воды по схеме Н2О = Н + + ОН –. Ионное произведение воды является постоянной величиной и зависит только от температуры раствора. Если раствор имеет комнатную температуру, то при расчетах K Впринимают равной 1×10–14.
Концентрации Н + и ОН – принято выражать в логарифмических единицах, в виде так называемых водородного показателя рНи гидроксильного показателя рОН
рН = – lg а  = –lg[Н+], (39)
= –lg[Н+], (39)
рОН = – lg а  = – lg[ОН–], (40)
= – lg[ОН–], (40)
Из уравнения (38) с учетом формул (39) и (40) следует, что
рН + рОН = 14. (41)
По значению рН растворы принято делить на три группы:
1) кислые –рН < 7; 2) щелочные – рН > 7; 3) нейтральныео – рН = 7.
Растворы, содержащие слабый электролит и его соль, способны поддерживать постоянным значение рН раствора. Такие растворы называют буферными. Их можно разделить на два типа: кислотный буфер – состоит из слабой кислоты и её соли; основной буфер – состоит из слабого основания и его соли.
Расчет концентрации ионов Н + в кислотном буфере следует вести по формуле
[Н+] =  , (42)
, (42)
где К Д(кислоты) – константа диссоциации слабой кислоты; [кислоты], [соли] – молярные концентрации кислоты и ее соли, моль/дм3.
Из формулы (42) следует, что
рН = р K Д(кислоты) – lg 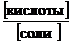 , (43)
, (43)
где р К Д(кислоты) = –lg К Д(кислоты).
По аналогии для буферной смеси слабого основания и его соли
рН = 14 – р K Д(основания) + lg  , (44)
, (44)
где р K Д(основания) = -lg K Д(основания).
Способность буферного раствора поддерживать постоянное значение рН определяется его буферной ёмкостью В, которая характеризуется количеством моль сильной кислоты или сильного основания, которое требуется ввести в 1 дм3 буферного раствора, чтобы изменить его рН на единицу. Буферная ёмкость выражается в моль/дм3 и определяется по формуле
 , (45)
, (45)
где С 2 – молярная концентрация сильной кислоты или основания; V – объём добавленного сильного электролита; VВ – объём буферного раствора; ΔpH – величина изменения рН раствора.
Добавление к буферному раствору кислоты понижает рН раствора, а прибавление щелочи увеличивает рН раствора. Буферная ёмкость раствора тем больше, чем выше концентрация его компонентов.
Примеры решения задач
Пример 1. Вычислите степень диссоциации, концентрацию ионов водорода и гидроксид ионов, водородный и гидроксильный показатель для 2 М раствора уксусной кислоты, если K д(СН3СООН) = 1,75×10-5.
Р е ш е н и е 


Уксусная кислота – слабый электролит, диссоциирующий по схеме СН3СООН Û СН3СОО– + Н+. K д(СН3СООН) << 1, поэтому для расчета степени диссоциации используем формулу (25)
a =  =
=  = 2,96×10-3 или 0,296 %
= 2,96×10-3 или 0,296 %
Концентрацию ионов водорода найдем по уравнению (20)
[Н+] =  = 5,92×10-3 моль/дм3.
= 5,92×10-3 моль/дм3.
Для определения концентрации ионов ОН– формулу (38) преобразуем к виду
[ОН–] =  =
=  = 1,69∙10-12 моль/дм3.
= 1,69∙10-12 моль/дм3.
Водородный и гидроксильный показатели рассчитываем по формулам (39) и (41)
рН = – lg[Н+] = –lg(5,92×10–3) = 2,23
рОН = 14 – рН = 14 – 2,23 = 11,77
Пример 2. Определите рН 0,017 М раствора муравьиной кислоты, если К Д(НСООН) = 2·10–4.
Р е ш е н и е
НСООН – слабая кислота, распадается на ионы по схеме НСООН Û НСОО– + Н+, поэтому расчет концентрации ионов Н+ ведем с использованием формул (25) и (20)
[Н+] = n × α· C 2= n ×  .
.
Водородный показатель рассчитываем по формуле (39)
рН = – lg 1,8 · 10-3 = 2,75.
Пример 3. Определите рН в растворе Са(ОН)2 с концентрацией 0,001 М.
Р е ш е н и е
Са(ОН)2 – щелочь, по первой ступени степень диссоциации α = 1, а по второй ступени степень диссоциации незначительная, поэтому ею можно пренебречь. Тогда схему диссоциации данного сильного электролита следует представить таким образом
Са(ОН)2 ® СаОН+ + ОН–.
Тогда [ОН–] = [Са(ОН)2] = 1×10-3. Данный раствор является сильно разбавленным, поэтому при расчете рОН можно использовать не активность, а концентрацию гидроксид-ионов. По уравнению (40)
рОН = – lg [ОН–] = – lg(1×10–3) = 3.
Из формулы (41) рН = 14 – рОН = 14 – 3 = 11.
Пример 4. Вычислите рН 0,1 М раствора азотной кислоты.
Р е ш е н и е
Азотная кислота – сильная, в растворе практически полностью диссоциирует на ионы по схеме HNO3 ® H+ + NO  . Степень диссоциации α ≈ 1, следовательно, [Н+] =[NO
. Степень диссоциации α ≈ 1, следовательно, [Н+] =[NO  ] = С 2(НNO3) = 0,1 моль/дм3. Поскольку концентрация кислоты достаточно велика, при расчете рН следует пользоваться активностью ионов водорода. Тогда по формуле (32)
] = С 2(НNO3) = 0,1 моль/дм3. Поскольку концентрация кислоты достаточно велика, при расчете рН следует пользоваться активностью ионов водорода. Тогда по формуле (32)
I = ½ × (0,1·12 + 0,1 · 12) = 0,1.
По уравнению (31 а) вычисляем коэффициент активности Н+
lgg+ = – 0,5×½(+1)2½×  = –0,158; g+ = 10–0,158 = 0,695.
= –0,158; g+ = 10–0,158 = 0,695.
По формулам (26) и (39)
а  = [Н+] ×g+ = 0,1 ×0,695 = 0,0695 М.
= [Н+] ×g+ = 0,1 ×0,695 = 0,0695 М.
рН = – lg а  = – lg 0,0695 = 1,16.
= – lg 0,0695 = 1,16.
Пример 5. Рассчитайте молярную концентрацию раствора азотистой кислоты НNO2, если рН = 2,7.
Р е ш е н и е
Азотистая кислота – слабый электролит, диссоциирует по схеме
НNO2 Û Н+ + NO 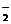
Из уравнения видно, что [Н+] = [NO 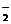 ]. Концентрации ионов найдем из формулы (39)
]. Концентрации ионов найдем из формулы (39)
[Н+] = [NO 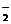 ] = 10–рН = 10–2,7 = 2×10–3 моль/дм3.
] = 10–рН = 10–2,7 = 2×10–3 моль/дм3.
Концентрация молекул кислоты, распавшихся на ионы, также составляет 2×10–3 моль/дм3. Обозначив исходную концентрацию кислоты через С2 и учитывая, что в начальный момент продуктов диссоциации не было, находим, что в состоянии равновесия концентрация недиссоциированных молекул кислоты равна [НNO2] = (С 2 − 2×10–3) М.
По формуле (22) константа диссоциации азотистой кислоты
 .
.
Значение К Д для азотистой кислоты взяли по справочнику [8]. Подставив в последнее выражение все известные концентрации, решаем уравнение относительно С 2
 ; С 2 = 0,01 М.
; С 2 = 0,01 М.
Пример 6. Рассчитайте концентрацию основания Ва(ОН)2 в растворе с рН = 11,3. Коэффициент активности γ (ОН−) = 0,965.
Р е ш е н и е
Ва(ОН)2 – щелочь, сильный электролит, диссоциирует по схеме Ва(ОН)2 → Ва2+ + 2ОН−. Из формулы (41)
рОН = 14 – рН = 14 – 11,3 = 2,7.
Активность ионов ОН− определим из уравнения (40)
 моль/дм3.
моль/дм3.
Из соотношения (26) находим концентрацию ионов ОН−
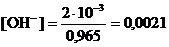 моль/дм3.
моль/дм3.
Из схемы диссоциации следует, что
С2 (Ва(ОН)2) = 1/2×[ОН−] = 0,0021/2=0,00105 моль/дм3.
Пример 7. Рассчитайте рН буферного раствора, содержащего в 1 дм3 0,1 моль СН3СООН и 0,2 моль NaСН3СОО. Оцените буферную ёмкость данного раствора по отношению к НСl.
Р е ш е н и е
По справочнику [8] находим значение константы диссоциации уксусной кислоты К Д(СН3СООН) = 1,75 · 10–5. Тогда
р К Д(СН3СООН) = –lg К Д(СН3СООН) = –lg(1,75 · 10–5) = 4,757.
По формуле (43) определяем рН раствора
рН = 4,757 – lg  = 5,058.
= 5,058.
При добавлении кислоты рН буферного раствора будет уменьшаться. Обозначим буферную ёмкость раствора по отношению к НСl через х моль. Тогда при добавлении х моль НСl к 1 дм3 раствора пойдет реакция
NaСН3СОО + НСl = NaСl + СН3СООН.
Концентрация СН3СООН увеличится до (0,1 + х) моль/л, а концентрация NaСН3СОО уменьшиться до (0,2 – х) моль/л.
При добавлении НСl в количестве, равном буферной ёмкости раствора, рН понизится на единицу, т. е. будет равным 4,058. В этом случае из (39)
[Н+] = 10–4,058 = 8,75·10–5.
По формуле (42)
[Н+] =  или 8,75 · 10-5 =
или 8,75 · 10-5 =  ;
;
 ; х = 6,67 моль/дм3.
; х = 6,67 моль/дм3.
Буферная ёмкость раствора по отношению к НСl равна 6,67 моль/л.
Пример 8. К 100 мл крови для понижения рН от 7,36 до 7,00 необходимо добавить 3,6 мл соляной кислоты с концентрацией 0,1 М. Чему равна буферная ёмкость крови по кислоте?
Р е ш е н и е
По формуле (45) найдем буферную ёмкость раствора по кислоте
 =
=  моль/л.
моль/л.






