C2H4 Ц C4H8 Ц газы, C5H10 Ц C16H32 - жидкости, C17H34 Ц Е Ц твердые вещества. јлкены плохо растворимы в воде. »х температуры плавлени€ и кипени€ закономерно повышаютс€ при увеличении молекул€рной массы соединени€.
6. ’имические свойства.
I. –еакции присоединени€.
1. гидрирование
1 2 3
CH2=CH Ц CH3 + H Ц H → CH3 Ц CH2 Ц CH3
пропилен пропан
2. галогенирование
1 2 3
CH2=CH Ц CH3 + Br Ц Br → CH2 Ц CH Ц CH3
| |
Br Br
пропилен 1,2-дибромпропан
3. гидрогалогенирование (по правилу ћарковникова: при присоединении веществ с пол€рной ковалентной св€зью типа HX (где X Ц это -Hal, -OH и т.д.) к несимметричным непредельным углеводородам по месту разрыва ѕ-св€зи атом водорода присоедин€етс€ к наиболее гидрированному атому углерода, а X Ц к наименее гидрированному атому углерода)
1 2 3
CH2=CH Ц CH3 + H Ц Br → CH3 Ц CH Ц CH3
|
Br
пропилен 2- бромпропан
4. гидратаци€ (по правилу ћарковникова) с образованием вторичных спиртов (кроме этилена Ц у него образуетс€ первичный спирт)
1 2 3
CH2=CH Ц CH3 + H Ц OH → CH2 Ц CH Ц CH3
|
OH
пропилен пропанол-2 (вторичный пропанол)
5. сульфирование (по правилу ћарковникова) с образованием алкилсерных кислот
1 2 3
CH2=CH Ц CH3 + HЦO Ц SO2OH → CH3 Ц CH Ц CH3
|
O Ц SO2OH
пропилен пропил-2серна€ кислота
CH3 Ц CH Ц CH3 + HЦOH → CH3 Ц CH Ц CH3 + HO Ц SO2 Ц OH
| |
O Ц SO2OH OH
пропил2серна€ кислота пропанол-2 серна€ кислота
6. алкилирование
CH3 CH3 CH3 CH3
| | | |
CH3 Ц CH + CH2=C Ц CH3 → CH3 Ц C Ц CH2 Ц CH Ц CH3
| |
CH3 CH3
метилпропан метилпропен 2,2,4-триметилпентан (изооктан)
7. взаимное алкилирование (обратный процесс крекинга алкенов), при разных температурах образуютс€ разные соединени€
CH2=CH Ц CH2 Ц CH3 бутилен-1
CH3 Ц CH=CH Ц CH3 бутилен-2
Kt, t0
CH2=CH2 + CH2=CH2 ЦЦ→ CH2=C Ц CH3 метилпропилен
|
CH3
CH2=CH Ц CH=CH2 + H2 бутадиен-1,3
II. –еакции окислени€.
1. горение
t0
C2H4 + 3 O2 (избыток) → 2 CO2 + 2 H2O
t0
C2H4 + 2 O2 (недостаток) → 2 CO + 2 H2O
t0
C2H4 + O2 (сильный недостаток) → 2 C + 2 H2O
2. частичное окисление кислородом воздуха с образованием эпоксидов (реакци€ ѕрилежаева)
t0, Ag
2 CH2=CH2 + O2 ЦЦ→2 CH2 Ц CH2
\ /
O
Ётиленоксид (эпоксиэтан или окись этилена)
3. окисление кислородом окислител€ в щелочной среде (реакци€ ¬агнера)
из KMnO4
3 CH2=CH2 + [O] + H Ц OH ЦЦЦЦЦЦЦЦ→ HO Ц CH2 Ц CH2 Ц OH
этиленгликоль (этандиол-1,2)
KOH
3 C-2H2=C-2H2 + 2 KMn+7O4 + 4 H2O ЦЦ→ 3 C-1H2 Ц C-1H2 + 2 Mn+4O2 + 2 KOH
| |
OH OH


 C2-2 -2ē → C2-1 3 3 C2-2 -6ē → 3 C2-1
C2-2 -2ē → C2-1 3 3 C2-2 -6ē → 3 C2-1
Mn+7 + 3ē → Mn+4 2 2 Mn+7 + 6ē → 2 Mn+4
ќбесцвечивание щелочного раствора KMnO4 Ц это качественна€ реакци€ на непредельные углеводороды.
4. жесткое окисление кислородом более энергичного окислител€ в кислой среде (кислый раствор KMnO4, HNO3, хромова€ смесь) при нагревании
|
|
|
+H3O+, t∞
R Ц CH=CH Ц R¢ + 4 [O] ЦЦ→ R Ц COOH + R¢ - COOH
—H3 Ц —H=CH Ц CH3 + 4 [O] ЦЦ→ 2 CH3 Ц COOH
бутилен-2 этанова€ (уксусна€) кислота
CH3 Ц CH2 Ц CH=CH2 + 4 [O] ЦЦ→ CH3 Ц CH2 Ц COOH + HCOOH
бутилен-1 пропанова€ метанова€ (муравьина€)
кислота кислота

 HCOOH + [O] ЦЦà HOCOOH (H2CO3)
HCOOH + [O] ЦЦà HOCOOH (H2CO3)
метанова€ CO2↑ H2O
кислота угольна€ кислота
CH3 Ц CH2 Ц CH=CH Ц CH3 + 4 [O] ЦЦ→ CH3 Ц CH2 Ц COOH + CH3 Ц COOH
пентен-2 пропанова€ кислота этанова€ кислота
5 CH3ЦCH2ЦC-1H=C-1HЦCH3 + 8 KMn+7O4 + 12 H2SO4 →4 K2SO4 + 8 Mn+2 SO4 + 12 H2O +
пентен-2
+ 5 CH3ЦCH2ЦC+3OOH + 5 CH3ЦC+3OOH
пропанова€ кислота этанова€ кислота


 C2-1 -8ē → 2 C+3 5 5 C2-2 -40ē → 10 C-1
C2-1 -8ē → 2 C+3 5 5 C2-2 -40ē → 10 C-1
Mn+7 + 5ē → Mn+2 8 8 Mn+7 + 40ē → 8 Mn+4
CH3 Ц CH2 Ц CH2 Ц CH=CH2 + 5 [O] ЦЦ→ CH3 Ц CH2 Ц CH2 Ц COOH + H2O + CO2
пентен-1 бутанова€ кислота
CH3 Ц CH2 Ц CH2 Ц C-1H=C-2H2 + 2 KMn+7O4 + 3 H2SO4 → K2SO4 + 2 Mn+2 SO4 + 4 H2O +
пентен-1
+ CH3 Ц CH2 Ц CH2 Ц C+3OOH + C+4O2


 бутанова€ кислота
бутанова€ кислота
 C-1 Ц 4ē → C+3 C-1 -4ē → C+3
C-1 Ц 4ē → C+3 C-1 -4ē → C+3
-10ē 1
C-2 Ц 6ē → C+4 C-2 -6ē → C+4
Mn+7 + 5ē → Mn+2 2 2 Mn+7 + 10ē → 2 Mn+2
III. –еакции полимеризации.
t0, kt, P
Е+ CH2=CH2 + CH2=CH2 + ЕЦЦЦЦЦЦ→ Е-CH2 Ц CH2 Ц CH2 Ц CH2-Е
этилен полиэтилен
t0, kt, P
nCH2=CH2 ЦЦЦЦЦЦ→ (Ц CH2 Ц CH2 Ц)n
мономер структурное звено
где n Ц это степень полимеризации
t0, kt, P
n CH3 Ц CH=CH2 ЦЦЦЦЦЦЦ→ (Ц CH Ц CH2 Ц)n
|
CH3
пропилен полипропилен
IV. –еакции изомеризации.
550∞C
CH2=CH2 Ц CH2 Ц CH3 ЦЦЦЦ→ CH3 Ц CH = CH Ц CH3 + CH3 Ц C = CH2
|
CH3
бутен-1 бутен-2 метилпропен
V. –еакции отщеплени€ (элиминировани€)
а) до алкадиенов:
MgO, ZnO
CH2=CH Ц CH2 Ц CH3 ЦЦЦЦ→ CH2 = CH Ц CH = CH2 + H2
бутен-1 бутадиен-1,3
б) до алкинов:
1200∞C
2 CH2=CH2 ЦЦЦЦ→ 2 CH≡CH + 2 H2
этен (этилен) этин (ацетилен)
ѕолучение алкенов.
1. рекинг нефтепродуктов
C16H34 → C8H18 + C8H16
гексадекан октан октен
ќбычно образуетс€ смесь различных углеводородов: например, при крекинге бутана конечными продуктами будет Ц смесь бутенов, пропилена, этилена и метана; при крекинге пропана Ц смесь пропилена, этилена и метана; при крекинге метилпропана Ц смесь метилпропилена, пропилена и метана.
2. ƒегидрирование предельных углеводородов
 CH2 = CH Ц CH2 Ц CH3 бутен-1
CH2 = CH Ц CH2 Ц CH3 бутен-1
CH3 Ц CH2 Ц CH2 Ц CH3 →
бутан CH3 Ц CH = CH Ц CH3 бутен-2
550-650oc, Kt
2CH4 ЦЦЦЦЦЦЦЦЦЦЦ→ C2H4 + 2H2
3. ¬нутримолекул€рна€ дегидратаци€ спиртов
H2SO4, 140-150oC
CH3 Ц CH2 Ц OH ЦЦЦЦЦЦЦЦЦЦЦЦЦЦЦЦ→ CH2 = CH2 + H2O
этанол этилен
4. ƒегидрогалогенирование галогенпроизводных алканов (по правилу «айцева: при отщеплении галогенводорода от вторичных и третичных галогеналканов атом водорода отщепл€етс€ о наименее гидрированного атома углерода)
Br
| спирт, to
CH3 Ц CH Ц CH2 Ц CH3 + NaOH ЦЦЦЦЦЦЦ→ CH3 Ц CH = CH Ц CH3 + NaBr + H2O
2-бромбутан бутен-2
5. ƒегалогенирование дигалогенпроизводных алканов
Br Br
| |
CH3 Ц CH Ц CH Ц CH3 + Zn → CH3 Ц CH = CH Ц CH3 + ZnBr2
2.3-дибромбутан бутен-2
ѕрименение алкенов
|
|
|
1. ѕрименение этилена
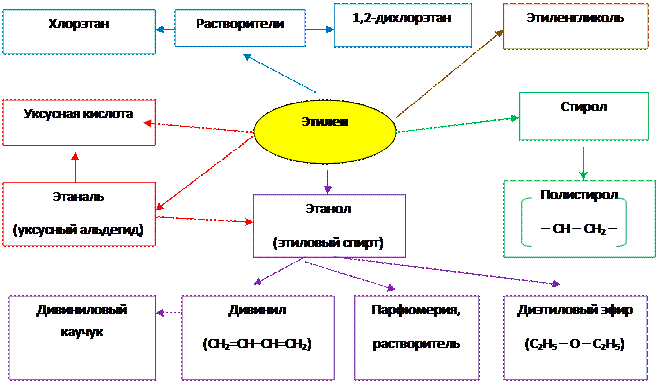 |
2. ѕрименение пропилена
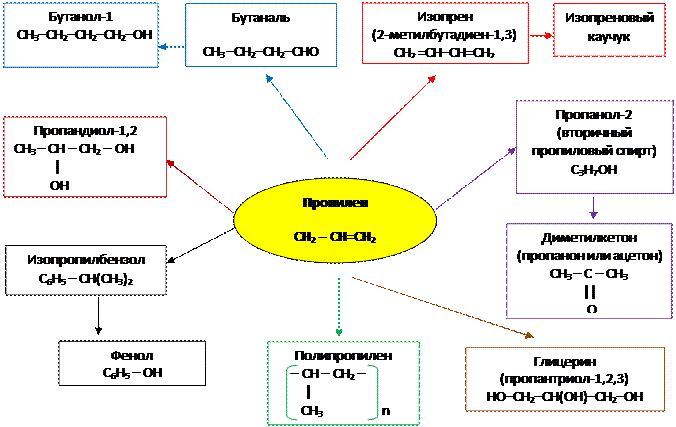
3. ѕрименение изомеров бутилена
| |||||
| |||||
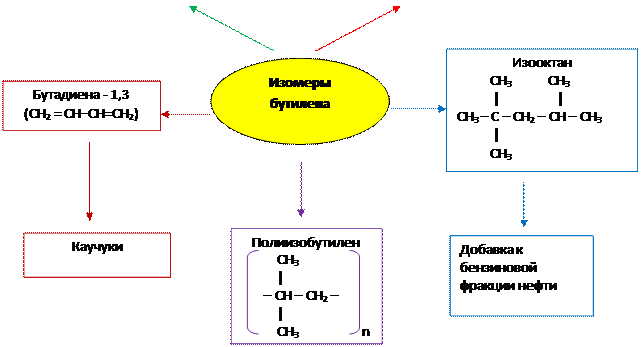 |
¬опросы: (дл€ контрол€ знаний)
1. акие соединени€ относ€тс€ к алкенам?
2. акое строение имеет молекулы алкенов (гибридизаци€, угол между гибридными орбитал€ми, длина св€зи)?
3. ак по правилами »ёѕј образуетс€ название неразветвлЄнного и разветвлЄнного алкена?
4. акие виды изомерии характерны дл€ алкенов?
5. ¬ какие реакции присоединени€ может вступить пропен? Ќапишите уравнени€ реакций.
6. ¬ какие реакции элиминировани€ может вступать алкены? Ќапишите уравнени€ реакций.
7. ¬ какие реакции окислени€ может вступать алкены? Ќапишите уравнени€ реакций.
8. акими способами можно получить алкены? Ќапишите уравнени€ реакций.
9. акое применение нашли алкены?
—писок используемых источников:
1. Ђ–епетитор по химии (издание 15-ое)ї, под редакцией ≈горова ј. —., ‘еникс Ц –остов-на-ƒону, 2006
2. √абриел€н ќ. —., ћаскаев ‘. Ќ., ѕономарев —. ё., “еренин ¬. ». Ђ’ими€ 10 класс: профильный уровеньї. (”чебник дл€ общеобразовательных учреждений), ƒрофа Ц ћосква, 2005
3. –удзитис √. ≈., ‘ельдман ‘. √. Ђ’ими€ 10: органическа€ хими€ (”чебник дл€ 10 класса средней школы)ї, ѕросвещение Ц ћосква, 1991






