1. Для раствора, содержащего 2,7·10-11 моль/л ионов H+, значение pH равно ________________ (10,57; 10,43; 11,57; 11,43).
2. Для раствора, содержащего 3·10-7 моль/л ионов H+, значение pH равно _________________(6,48; 7,48; 6,52; 7,52).
3. Концентрация гидроксид-ионов в водном растворе аммиак с РН = 10 составляет_________моль\дм3.
4. В растворе с рН=3.09 концентрация ионов водорода равна….
5. В растворе с РН=10,57 концентрация ионов водорода равна…
6. В растворе с pH = 5,7 концентрация ионов водорода равна…
7. Молярная концентрация эквивалентов сульфата меди (II) в растворе, полученном растворением 12,5 г медного купороса в 100 мл воды (изменением объема при растворении твердого вещества пренебречь), равна ___________ моль/л.
8. 500 см3 водного раствора, содержащего 106 г карбоната натрия, разбавили дистиллированной водой в два раза. Молярная концентрация карбоната натрия в полученном раствора составляет ________ моль/л.
9. Временная гидрокарбонатная жесткость, на удаление которой из 100 мл воды потребовалось 2,5 мл 0,1 н раствора HCl, составляет ____ мэкв/л.
10. Временная гидрокарбонатная жесткость, на удаление которой из 100 мл воды потребовалось 3 мл 0,1 н раствора HCl, составляет ____ мэкв/л.
11. Жесткость воды равна 2,5 мэкв/л соответствует содержанию Ca(HCO3)2 массой____________ в одном литре воды.
12. Жесткость воды равна 4,1 мэкв/л соответствует содержанию Ca(HCO3)2 массой_____ в одном литре воды.
13. Жесткость воды, равная 1,5 мэкв/л, соответствует содержанию CaHCO3 массой ____ мг в одном л воды.
14. Для устранения временной жесткости, равной 4,1 ммоль-экв/л, к 1000 л воды необходимо добавить ____ г Ca(OH)2 (Ответ приведите с точностью до целого значения.)
15. Для устранения временной жесткости, равной 3,6 ммоль-экв/л, к 1000 л воды необходимо добавить ____ г Ca(OH)2 (Ответ приведите с точностью до целого значения.)
16. Для устранения временной жесткости, равной 1,8 ммоль-экв/л, к 100 л воды необходимо добавить_______ г Са(ОН)2.
17. Для устранения временной жесткости, равной 2,4 ммоль-экв/л, к 100 л воды необходимо добавить_______ г Са(ОН)2.
19. Для осаждения ионов тяжелых металлов из сточных вод применяют Са(ОН)2. Если объем очищаемой воды равен 1000 м3, а С Мn2+ = 275 мг/дм3, то с учетом 10 %-ного избытка реагента, необходимого для осаждения, расход Са(ОН)2 составит______кг в год.
20. Для устранения кислого характера сточных растворов часто применяется известняковая мука. Если суточный объем очищаемой воды равен 1000 м3, значение рН исходного раствора равно 3, то с учетом 80 %-го содержания действующего вещества в пересчете на карбонат кальция в известняковой муке ее расход составит ______ кг в сутки.
21. Для устранения кислого характера сточных растворов часто применяется известняковая мука. Если суточный объем очищаемой воды равен 2000 м3, значение рН исходного раствора равно 4, то с учетом 80 % содержания действующего вещества в пересчете на карбонат кальция в известняковой муке ее расход составит ______ кг в сутки.
22. Для устранения кислого характера сточных растворов часто применяется известняковая мука. Если суточный объем очищаемой воды равен 500 м3, значение рН исходного раствора равно 2, то с учетом 80 %-го содержания действующего вещества в пересчете на карбонат кальция в известняковой муке ее расход составит ______ кг в сутки.
Электролиз
1. Установите последовательность восстановления металлов из раствора, содержащего равные концентрации их ионов: Fe+2; Sn+2; Ag+; Cu+2._______________________________
2. Установите последовательностьвосстановления металлов из раствора, содержащего равные концентрации их ионов: Pb+2; Sn+2; Ag+; Cd+2_______________________________
3. Установите последовательность восстановления металлов из раствора, содержащего равные концентрации их ионов: Au3+; Cu2+; Pb2+; Ni2+______________________________
4. Продуктами электролиза на инертных электродах раствора соли KBr являются___________________(H2 и O2; K и Br2; K и HBr, O2; H2, Br2, KOH).
5. Продуктами электролиза на инертных электродах раствора соли Pb(NO3)2 являются___________(H2 и O2; Pb и O2, HNO3; H2 и NO2, Pb(OH)2; Pb и O2).
6. Продуктами электролиза на инертных электродах раствора соли ZnCl2 является…
7. Продуктами электролиза на инертных электродах раствора соли NaNO3 являются…
8. Значение РН раствора увеличивается в результате электролиза с инертными электродами водного раствора соли, формула которой ______________ (CuCl2; Cu(NO3)2; NaCl; Na2SO4).
9. При электролизе раствора SnCl2 на инертном аноде выделилось 8,96 л хлора, а на катоде _____ г олова. (Ответ приведите с точностью до десятых.).
10. При электролизе раствора SnCl2 на инертном аноде выделилось 2,24 л хлора, а на катоде _____ г олова. (Ответ приведите с точностью до десятых.)
11. Если при электролизе AuCl3 током силой 6 А в течение 30 минут выделилось 6,5 г золота, выход по току составил ____%. (Ответ приведите с точностью до целого значения.
12. Если при электролизе AuCl3 током силой 4 А в течение 40 минут выделилось 6 г золота, то выход по току составил ____%. (Ответ приведите с точностью до целого значения.).
13. При электролизе водного раствора KOH силой тока 6 А в течение 30 минут выделилось ____ кислорода. (Ответ приведите с точностью до сотых.).
14. При электролизе водного раствора KOH силой тока 6 А в течение 20 минут выделилось ____ водорода. (Ответ приведите с точностью до сотых.).
15. Наиболее технологичным и эффективным способом выделения ценных металлов из растворов является электролиз. Если годовой объем очищаемой воды равен 1000 м3, а содержание в ней ионов Рt4+ в виде анионных комплексов составляет 1,0 мг/дм3, то время, необходимое для выделения всей платины электролизом при силе тока 22,9 А и выходе по току 80 %, составит ____ часов. (Ответ привести с точностью до целых; Ar(Pt) = 195; F = 96500 Кл/моль.).
16. Если годовой объем очищаемой воды равен 1000 м3, а содержание в ней ионов Pd2+ составляет 0,53 мг/дм3, то время, необходимое для выделения всего палладия электролизом при силе тока 22,3 А и выходе по току 80 %, составит ______ часов. (Ar (Pd)=106; F=96500 Кл/моль.).
17. Если годовой объем очищаемой воды равен 1000 м3, а содержание в ней ионов Pd2+ составляет 10.6 мг/дм3, то время, необходимое для выделения всего палладия электролизом при силе тока 22,3 А и выходе по току 80 %, составит ______ суток. (Ar (Pd)=106; F=96500 Кл/моль.)
18. Если годовой объем очищаемой воды равен 1500 м3, а содержание в ней ионов Ag+ составляет 20,16 мг/дм3, то время, необходимое для выделения всего серебра электролизом при силе тока 22,33 А и выходе по току 90 %, составит ______ суток. (Ar (Ag)=108; F=96500 Кл/моль.).
19. Масса Ag, выделившегося при электролизе раствора AgNO3 током силой 5 А в течение 20 минут и выходе по току 90 %, составляет___ г.
20. Масса Ag, выделившегося при электролизе раствора AgNO3 током силой 4 А в течение 35 минут и выходе по току 96 %, составляет___ г.
21. Наибольшее количества электричества потребуется для получения путем электролиза (выход по току принять 100 %) одного грамма____ Варианты ответа: Cd, Hg, Mg, Cu.
Пирометаллургия
1. Если значения стандартной энергии Гиббса образования в кДж/моль для оксидов равны 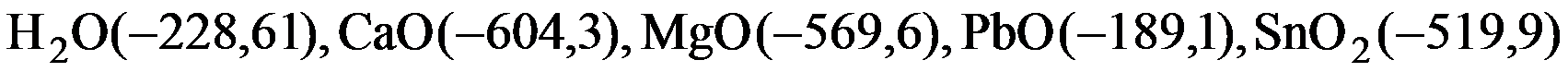 , то металлом, который можно восстановить водородом при стандартных условиях из оксида, является: Pb; Ca; Sn; Mg.
, то металлом, который можно восстановить водородом при стандартных условиях из оксида, является: Pb; Ca; Sn; Mg.
2. Если значения стандартной энергии Гиббса образования в кДж/моль для оксидов равны: 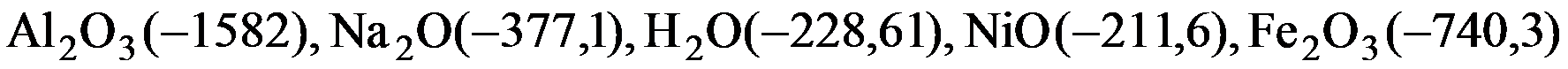 , то металлом, который можно восстановить водородом при стандартных условиях из оксида, является____________
, то металлом, который можно восстановить водородом при стандартных условиях из оксида, является____________
3. Значение стандартной энергии Гиббса образования в кДж/ моль для оксидов равны:
Al2O3 (-1582), NiO (-211,6), CuO (-129,4), Cr2O3 (-1059), CO2(-394,38). Из оксида можно восстановить углеродом при стандартных условиях металл____
4. Значение стандартной энергии Гиббса образования в кДж/ моль для оксидов равны:
Al2O3 (-1582), MgO(-569,6), CuO(-129,4), Cr2O3(-1059), Fe2O3(-740,3). Алюминотермией нельзя восстановить при стандартных условиях из оксида металл_______
5. Если для получения Pb используется реакция: C + PbO = Pb + CO + ∆H1, теплота, необходимая для осуществления процесса обеспечивается горением углерода: С + О2 = СО2 + ∆Н2, то для получения 828 кг Pb потребуется ______ кг углерода. Энтальпии образования веществ: ∆H PbO = - 218, ∆H CO = -111, ∆H = CO2 = -394 кДж/моль.






