The aim: determination of the Rydberg constant by spectroscope's method.
Instrumentation and appliances: monochromator YM-2, mercury lamp, hydrogen lamp.
A short theory
A hydrogen atom has one electron which "rotates" in a nuclear field. An electric force on Coulomb attraction acts between the electron and the nucleus. The potential energy of an electron in a nuclear field is
 , (11.1)
, (11.1)
where e is the charge of an electron and r is the distance between the nucleus and electron. Such an atom constitutes a peculiar kind of potential well and is illustrated in fig.11.1.

Figure 11.1
The electron inside the atom has a negative potential energy since the minimum value of potential energy tends to infinity when r → 0 and the maximum value is equal to zero. Fig. 11.2 shows the energy levels obtained from the solution of the Schrodinger equation
 . (11.2)
. (11.2)
 |
Figure 11.2
An important feature of the solution is the drawing together of the levels as the quantum number n increases. The scales of values, which are proportional to energy are given in the units adopted in spectroscopy: volts and reciprocal centimetres. The energy level formula may by written in the form
 . (11.3)
. (11.3)
For historical reasons, it is customary to write this formula in the for
 , (11.4)
, (11.4)
where
 (11.5)
(11.5)
is the RydbergТs constant.
The atomic electron may be located at any one of n levels. The energy of a free hydrogen atom on which no force acts is at the lowest energy level
 , (11.6)
, (11.6)
The energy ε = cRh is called the ionisation energy. If the energy imparted to hydrogen atom is less than cRh, a transition of the atom occurs to one of the n levels. Such an atom is said to be in an exited state.
An atom stays in an excited state for a small fraction of a second and then passes to a lower level with the emission off a photon in accordance with the equation
hνmn = εm - εn = cRh (1/n2 - 1/m2). (11.7)
By calculating for a given n the ν frequencies corresponding to the numbers m = n +1, n +2,..., we obtain a series of frequencies of lines in the hydrogen spectrum. The series corresponding to n = 2 is known as the Balmer series.

 . (11.8)
. (11.8)
The experimental part
1. Determine graduate graph of monochromator by mercury spectrum. For it you must to revolve the monochromator drum until you can see the spectrum line in micrometer eyepiece.
For mercury lamp:
| Colour of spectrum line | Wavelength, 10-9,m |
| Violet Blue Light blue Green Yellow | 410,805 434,749 435,833 491,607 502,564 546,073 578,966 |
2. Determine the line position of red, green and blue on monochromator drum of hydrogen spectrum.
3. Determine the wave length of red, green and blue lines of hydrogen spectrum used graduate graph of monochromator.
|
|
|
4. Calculate the Rydberg constant by formula
 , (11.9)
, (11.9)
where λmn is wavelength; n = 2 and m = 3 (red), 4 (dark blue), 5 (light blue), 6 (violet).
6. Put down the date of measurements in the table:
| n2 | Colour of line | Angle of rotation a monochromator dram | Wavelength, l, nm | Rydberg constant, R i, m-1 | 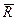 ,
m-1 ,
m-1
|
| Red | |||||
| Dark blue | |||||
| Light blue | |||||
| Violet |
6. Make analysis of the experiment results.
Control questions
1. What is dispersion of light?
2. What is physically meaning of coefficient refraction of light?
3. Formulate Bor's postulates.
4. What is dark-line spectrum and bright-line spectrum, continuous spectrum and discrete spectrum?
References
1. —ивухин ƒ. ¬. ќбщий курс физики. Ц т. 4. Ц ћ.: Ќаука, 1980.- с. 38 Ц 39.
2. —авельЇв ≤.¬. У урс загальноњ ф≥зикиФ, т.2.ћ.,1980.- с. 452 Ц 454.
3. —авельЇв ≤.¬. У урс загальноњ ф≥зикиФ, т.2.ћ.,1980.- с. 93 Ц 99.
4. ‘изический энциклопедический словарь.- ћ.: —Ё, 1999.- с. 702.
Authors: Lushchin S.P., the reader, candidate of physical and mathematical sciences.
Reviewer: Loskutov S.V., professor; doctor of physical and mathematical sciences.
12 ЋјЅќ–ј“ќ–Ќј –ќЅќ“ј є 62.1 ƒќ—Ћ≤ƒ∆≈ЌЌя я¬»ўј ≤Ќ“≈–‘≈–≈Ќ÷≤ѓ —¬≤“Ћј
( ќћѕТё“≈–Ќ»… ¬ј–≤јЌ“)
ћета роботи - досл≥дити €вище ≥нтерференц≥њ. ¬изначити довжину хвил≥ лазерного випром≥нюванн€.
ѕрилади ≥ обладнанн€: компТютер, компТютерна програма ФinteФ.
“еоретична частина
явище ≥нтерференц≥њ пол€гаЇ у перерозпод≥л≥ св≥тлового потоку у простор≥ при накладанн≥ когерентних хвиль. огерентними називаютьс€ хвил≥, €к≥ мають однакову частоту ≥ сталу у час≥ р≥зницю фаз. ≤нтерференц≥йна картина €вл€Ї собою чергуванн€ св≥тлих ≥ темних смуг (максимум≥в ≥ м≥н≥мум≥в). ¬≥дстань м≥ж двома сус≥дн≥ми максимумами визначаЇтьс€ по формул≥:
 , (12.1)
, (12.1)
де  - довжина хвил≥;
- довжина хвил≥;  - в≥дстань м≥ж щ≥линами та екраном;
- в≥дстань м≥ж щ≥линами та екраном; 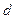 - в≥дстань м≥ж щ≥линами. « формули (12.1) дл€ довжини хвил≥ одержуЇмо:
- в≥дстань м≥ж щ≥линами. « формули (12.1) дл€ довжини хвил≥ одержуЇмо:
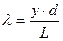 . (12.2)
. (12.2)
12.2 ’≥д роботи
a. «апустити компТютерну програму ФinteФ. ќзнайомитись з теор≥Їю до роботи.
b. ¬ибрати ф≥льтр шл€хом натискуванн€ рад≥о кнопки. –екомендуЇтьс€ вибрати спочатку ф≥льтр, €кий пропускаЇ випром≥нюванн€ з довжиною хвил≥ 640 нм.
c. ќтримати на екран≥ ≥нтерференц≥йну картину. Ќатиснути кнопку Ђмалюнокї, та на екран≥ намалюЇтьс€ в≥дпов≥дна обраному ф≥льтру ≥нтерференц≥йний малюнок.
d. ¬им≥р€ти л≥н≥йкою ширину ≥нтерференц≥йноњ картини в п≥ксел€х. «а допомогою л≥н≥йки вим≥р€йте сп≥льну ширину дек≥лькох ≥нтерференц≥йних смуг. –озд≥ливши на к≥льк≥сть смуг, отримуЇте ширину одн≥Їњ смуги. ¬им≥рюванн€ повторити дек≥лька раз≥в.
e. ѕовторити досл≥ди з ≥ншими ф≥льтрами, €к≥ пропускають випром≥нюванн€ з нев≥домими довжинами хвиль.
f. ¬изначити дожину хвил≥ дл€ кожного св≥тлового ф≥льтра. ѕо формулам дл€ схеми ёнга розрахуйте довжини хвиль дл€ 3 ф≥льтр≥в, враховуючи в≥дстань в≥д джерел до екрану та в≥дстань м≥ж пост≥йними джерелами.
λ / y = λ2 / y2 = λ3 / y3= const, (12.3)
тобто:
λ2 = λy2 /y. (12.4)
“ак €к L ≥ d - константи, то λ / y Ц стала величина. y - в≥дстань м≥ж сус≥дн≥ми максимумами, €ка в≥дзначаЇтьс€ за формулою:
|
|
|
yn = Yn / k. (12.5)
≥льк≥сть п≥кселей вим≥рюютьс€ екранною л≥н≥йкою. ¬им≥рюЇтьс€ одразу дек≥лька пром≥жк≥в м≥ж максимумами, п≥сл€ цього в≥дстань д≥литьс€ на цю к≥льк≥сть.
–езультати занести до таблиц≥:
| є | кол≥р | ≥льк≥сть п≥кселей | λ, довжина хвил≥, нм |
| червоний | |||
| син≥й | |||
| зелений | |||
| темно-червоний |
7. ѕоставити тонку пластину (товщина пластини d = 10мкм) на шл€ху одного з пучк≥в. ƒл€ наочност≥ необх≥дно обрати зелений св≥тлоф≥льтр, €кий при закритт≥ одного джерела скл€ною пластиною, в≥дхил€Їтьс€ на ц≥ле число максимум≥в зм≥щенн€.(—кл€на пластина зм≥щуЇтьс€ на екран≥ одночасним натисканн€м двох кнопок миш≥.
8. ѕоставити на шл€ху одного з св≥тлових пучк≥в пластинку, клацнувши по н≥й мишкою (повторно клацнувши на ц≥й пластин≥ вона приберетьс€ з оптичноњ схеми). ÷е призведе до по€ви додатковоњ оптичноњ р≥зниц≥ ходи, викликаною в≥дм≥нн≥стю показника заломленн€ пластинки в≥д одиниц≥. “овщина пластинки 10 мкм.
9. ¬изначити показник заломленн€ ≥з сп≥вв≥дношенн€:
(n-1)d=l1-l2
(n-1)d= k λ
n=1+ k λ / d, (12.6)
де k- к≥льк≥сть максимум≥в зм≥щенн€.
10. ¬изначити наск≥льки смуг зм≥ститьс€ п≥сл€ введенн€ пластинки ≥нтерференц≥йний малюнок (дл€ цього пор€д знаходитьс€ не зм≥щений малюнок). «а теоретичною формулою визначте показник заломленн€ речовини. «а вказ≥вками викладача цей досл≥д можна повторити дл€ ≥нших св≥тлоф≥льтр≥в.
11. ѕоставити товсту пластину на шл€ху одного з пучк≥в., визначте зм≥щенн€ ≥нтерференц≥йних малюнк≥в.
12. ¬изначити товщину пластини. ¬изначивши у попередньому досл≥д≥ показник заломленн€ речовини пластин, визначте товщину товстоњ пластинки.
13. «робити висновки. ¬≥дм≥тьте у висновках можлив≥сть вим≥рюванн€ показника заломленн€ речовин.
онтрольн≥ запитанн€
1. ¬ чому пол€гаЇ €вище ≥нтерференц≥њ св≥тла?
2. як≥ джерела хвиль називають когерентними?
3. ўо таке оптична р≥зниц€ ходу?
4. якими формулами визначаютьс€ умови ≥нтерференц≥йного максимуму та м≥н≥муму?
Ћ≥тература
1. —авельЇв ≤. ¬. урс загальноњ ф≥зики т. 3, с. 78-79, 81-85.
2. яворський ¬. ћ., ƒетлаф ј. ј. урс загальноњ ф≥зики т. 3.
3. ‘р≥ш —. ≈., “≥мофеЇв ј. ¬. урс загальноњ ф≥зики т. 3, с. 36-40
ћетодичн≥ вказ≥вки розробив доц. —ерпецький Ѕ.ќ.
–ецензент доц. ѕравда ћ. ≤.






