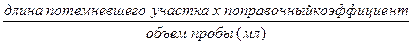1. Рассчитать содержание извести (в фунтах/баррель).
Известь (фунт/барр) = Pom х 1,295
Известь (фунт/барр) = 3,0 х 1,295 = 3,89
2. Рассчитать содержание кальция CaCl2БР в мг/л.
CaCl2БР = 2,774 х СаБР
CaCl2БР = 2,774 х 33 600 = 93 206 мг/л
3. Рассчитать концентрацию (в фунтах/барр) CaCl2.
СaCl2(фунт/барр) = 0,000971 х СаБР
СaCl2(фунт/барр) = 0,000971 х 33 600 = 32,6 фунт/барр
4. Рассчитать концентрацию хлорид-ионов, ассоциированных в CaCl2, в мг Cl- на 1 л бурового раствора.
СlCaCl2 = 1,77 х СаБР
СlCaCl2 = 1,77 х 33 600 = 59 472 мг/л
5. Если СlCaCl2 больше или равно СlБР, предполагается, что в растворе содержится только соль CaCl2, а сам раствор не является двухсолевой системой. Если СlCaCl меньше, чем СlБР, предполагается, что в буровом растворе содержатся соли CaCl2 и NaCl (иными словами, раствор является двухсолевым). Далее можно продолжить расчеты.
6. Рассчитать концентрацию хлорид-ионов, ассоциированных с NaCl в мг Cl- соли NaCl на 1 л бурового раствора.
ClNaCl = ClБР - ClCaCl2
ClNaCl = 100 000 – 59 472 = 40 528 мг/л
7. Рассчитать содержание NaCl в буровом растворе в мг соли NaCl на 1 л бурового раствора.
NaClБР = 1,65 х ClNaCl
NaClБР = 1,65 х 40 528 = 66 871
8. Рассчитать концентрацию NaCl в фунтах/баррель.
NaClфунт/барр. = 0,00035(NaClБР)
NaClфунт/барр. = 0,00035 х 66 871 = 23,4 фунта/баррель
Расчеты для определения минерализации водной фазы
9. Рассчитать весовой процент CaCl2 (вес%CaCl2).
Вес%CaCl2 = 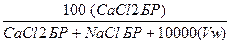
Вес%СaCl2 = 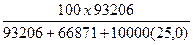 = 22,73% веса
= 22,73% веса
10. Рассчитать весовой процент NaCl (вес%NaCl).
Вес%NaCl = 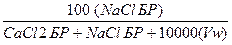
Вес%NaCl = 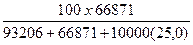 = 16,31% веса
= 16,31% веса
11. Используя формулу для расчетов взаимной растворимости для растворов солей NaCl и CaCl2, рассчитать максимальное количество растворимой соли NaCl (NaClmax).
NaClmax = 26,432 – 1,0472(вес%CaCl2) + 7,98191 х 10-3(вес%CaCl2) +
+ 5,2238 х 10-5(вес%CaCl2)3
NaClmax = 26,432 – 1,0472 х 22,73 + 7,98191 х 10-3 х 22,732 + 5,2238 х 10-5 х 22,732 = 7,37
Если рассчитанное максимальное количество растворимой соли NaCl (NaClmax) из формулы №11 меньше, чем рассчитанный процентный вес NaCl из формулы №10, результат расчетов содержания солей в водной фазе бурового раствора по формулам №9 и №10 ошибочен, т.к. фактически в растворе присутствует не все количество соли NaCl.
12. Необходимо рассчитать NaClmax заново при помощи коэффициента:
Коэффициент = 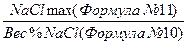
Коэффициент = 7,37 / 16,31 = 0,4519
13. Новое значение NaClmax = Старое значение NaClmax х Коэффициент
Новое значение NaClmax = 66 871 х 0,4519 = 30 219 мг/л.
14. Заменив старые значения NaClmax новыми, повторить расчеты по формулам №9 и №10.
Вес%CaCl2 = 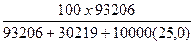 = 24,96 % веса
= 24,96 % веса
Вес%NaCl = 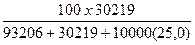 = 8,09 % веса
= 8,09 % веса
15. Используя новые значения Вес%CaCl2 и Вес%NaCl, рассчитать новое значение NaClmax (формула №11).
NaClmax = 26,432 – 1,0472 х 24,96 + 7,98191 х 10-3 х 24,962 + 5,2238 х 10-5 х 24,963 = 6,08
16. Рассчитать новый коэффициент (формула №12).
Коэффициент = 6,08 / 8,09 = 0,7515
17. Продолжить расчеты (формулы №11 - №16) до тех пор, пока коэффициент не превысит 0,95.
Новое значение NaClБР = Старое значение NaClБР х Коффициент
Новое значение NaClБР = 30 219 х 0,7515 = 22 710 мг/л
Вес%CaCl2 = 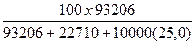 = 25,47 % веса
= 25,47 % веса
Вес%NaCl = 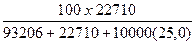 = 6,21 % веса
= 6,21 % веса
NaClmax = 26,432 – 1,0472 х 25,47 + 7,98191 х 10-3 х 25,472 + 5,2238 х 10-5 х 25,473 = 5,80
Коэффициент = 5,80 / 6,21 = 0,934
Новое значение NaClБР = 22 710 х 0,934 = 21 211 мг/л
Вес%CaCl2 = 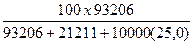 = 25,58 % веса
= 25,58 % веса
Вес%NaCl = 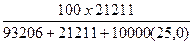 = 5,82 % веса
= 5,82 % веса
NaClmax = 26,432 – 1,0472 х 25,58 + 7,98191 х 10-3 х 25,582 + 5,2238 х 10-5 х 25,583 = 5,74
Коэффициент = 5,74 / 5,82 = 0,986
18. Рассчитать концентрацию CaCl2 в промилле (ppm).
CaCl2(ppm) = 10 000 х конечный вес%CaCl2
CaCl2(ppm) = 10 000 х 25,58 = 255 800
19. Рассчитать концентрацию NaCl в промилле (ppm).
NaCl(ppm) = 10 000 х конечный вес%NaCl
NaCl(ppm) = 10 000 х 5,82 = 58 200
20. Рассчитать плотность бурового раствора (γБР), выраженную через его удельный вес.
γБР = 0,99707 + 0,006504 (весNaCl) + 0,007923 (весCaCl2) + 0,00008334(весNaCl)(весCaCl2) + 0,00004395(весNaCl)2+ 0,00004964(весCaCl2)2
γБР = 0,99707 + 0,006504 (5,82) + 0,007923 (25,58) + 0,00008334 (5,82)(25,58) + 0,00004395 (5,82)2 + 0,00004964(25,58)2.
γБР = 1,28 г/см3
21. Рассчитать концентрацию CaCl2 в мг/л.
CaCl2(мг/л) = 10 000 (весCaCl)(γБР)
CaCl2(мг/л) = 10 000 (25,58)(1,28) = 327 424 мг/л
22. Рассчитать концентрацию NaCl в мг/л.
NaCl(мг/л) = 10 000 (весNaCl)(γБР)
NaCl(мг/л) = 10 000 (5,82)(1,28) = 74 496 мг/л.
D) СУЛЬФИДЫ
Описываемая ниже процедура выполняется при измерении концентрации химически активных растворимых сульфидов в буровом растворе на углеводородной основе.
 Оборудование и реагенты
Оборудование и реагенты
1. Газоанализатор Гарретта в сборе.
2. Индикаторные трубки Дрегера для определения низких и высоких концентраций сероводорода.
3. Расходомер.
4. Эластичная трубка из латекса или тайгона (или аналогичных материалов).
5. Магнитная мешалка.
6. Перемешиватель магнитной мешалки (1/4 х 1 дюйм).
7. Шпирц емкостью 10 мл, стеклянный или пластмассовый.
8. 2-мольный раствор лимонной кислоты/изопропилового спирта/деэмульгатора.
а. 420 г лимонной кислоты (C6H8O7H2O) растворить в 1 000 мл деионизированной воды.
b. к кислотному раствору добавить 25 мл деэмульгатора Dowell W-35 от компании Fann Instrument или аналогичный деэмульгатор.
с. к полученному раствору добавить 200 мл изопропилового спирта.
9. Пеногаситель: октанол (CAS # 111-87-5).
10. Градуированный стакан емкостью 25 мл.
11. Газ: СО2 в баллонах, рекомендуется азот. N2O не применять!
12. 16-дюймовая (15,2 мм) трубка для ввода проб из тефлона или аналогичного материала.
Процедура
1. Очистить и высушить газоанализатор Гарретта.
2. В резиновую мембрану газоанализатора вставить трубку для ввода проб. Нижний конец трубки должен находится на расстоянии приблизительно ½ дюйма от дна камеры 1 газоанализатора. Трубка предназначена для введения пробы непосредственно в раствор лимонной кислоты.
3. Установить магнитную мешалку в камеру 1, палочка мешалки должна свободно вращаться вокруг своей оси.
4. Вставить баллон с углекислым газом.
5. В камеру 1 налить 20 мл 2-мольного раствора лимонной кислоты/изопропилового спирта/деэмульгатора.
6. В камеру 1 добавить 10 капель пеногасителя (октанол).
7. Отломив запаянные концы трубки Дрегера, вставить трубку в держатель газоанализатора, расположенный справа. Стрелка, обозначающая направление потока, должна указывать вниз. Вставить уплотнительное кольцо на трубку Дрегера.
8. Вставить расходомер в кронштейн стрелкой вверх. Вставить уплотнительное кольцо.
9. Установить верхний блок газоанализатора, затянуть крепежные винты.
10. Отрегулировать положение дисперсионной трубки так, чтобы она находилась выше уровня жидкости.
11. Соединить резиновый шланг от регулятора с дисперсионной трубкой. Соединить резиновым шлангом трубку Дрегера и камеру 3. ГАЗ НЕ ПОДАВАТЬ!
12. В шприц набрать необходимый объем бурового раствора и дополнительно 0,5 мл, так как часть пробы раствора останется в трубке для ввода пробы.
13. Вставить шприц в трубку для ввода пробы. Зафиксировать поршень шприца резиновым жгутом, чтобы предотвратить попадание смеси лимонной кислоты и пеногасителя в трубку при подаче давления.
14. Для продувки газоанализатора подавать газ под небольшим давлением через дисперсионную трубку в течение 10 – 15 секунд. Отрегулировать расход газа таким образом, чтобы раствор в камере 1 не вспенивался. Проверить герметичность соединений расходомера и его работоспособность.
15. Включить магнитную мешалку. Регулируя скорость, добиться образования воронки. Аккуратно опустить дисперсионную трубку в жидкость так, чтобы конец трубки был слегка выше лопасти мешалки.
16. Опустить трубку ввода пробы так, чтобы ее конец был немного ниже, чем конец дисперсионной трубки. При таком расположении трубок буровой раствор будет сразу же попадать в вихревую воронку.
17. Медленно ввести отмеренное количество бурового раствора (см. Табл. 6) в камеру 1 через трубку для ввода раствора. Увеличить скорость работы мешалки, чтобы предотвратить налипание бурового раствора на стенки камеры. Продолжать перемешивание в течение 1 минуты.
18. Зафиксировать поршень шприца при помощи резинового жгута. Открыть подачу газа. Расход газа должен находиться в пределах от 200 до 400 мл/мин (шарик расходомера будет находится между красными отметками).
19. Наблюдать изменение цвета трубки Дрегера. Измерить и записать максимально различимую длину потемневшего участка (в единицах измерения, указанных на маркировке трубки) до того, как пятно в передней части трубки начнет расплываться или размазываться. Продолжать подачу газа. Общее время подачи газа должно составить 15 минут, поэтому может понадобиться второй газовый баллон.
20. Во избежание повреждения пластиковых деталей газоанализатора произвести его очистку сразу же по окончании анализа. Промыть камеры и каналы между ними теплой водой со слабым моющим средством. Промыть дисперсионную и вводную трубки органическим растворителем (например, ацетоном), вымыть остатки растворителя водой. Иногда для очистки дисперсионной трубки от карбонатных отложений ее необходимо обработать в кислотной ванне. Органические растворители могут вызвать растрескивание корпуса газоанализатора. Содержание сульфидов в буровом растворе рассчитывается исходя из объема пробы, длины потемневшего участка индикаторной трубки Дрегера и поправочного коэффициента индикаторной трубки.
Содержание сульфидов (мг/л) =