¬ наст. врем€ невозможно рассчитать или экспериментально определить абсолютное значение скачка потенциала на отдельно вз€том электроде. ѕоэтому значение потенциала на электроде всегда определ€етс€ по отношению к эталонному электроду, потенциал к-го условно принимаетс€ =0.¬ электрохимии таким электродом €вл€етс€ нормальный водородный элемент (н.в.э.).ѕотенциал н.в.э. при опред-х услови€х =0. ¬ стекл€нный сосуд сложной формы залит раствор серной кислоты такой концентрации, что [H+]ї1 г-ион/л.ѕлатинова€ чернь (губчата€ пластина) Ц платиновый электрод.–=1атм.¬одород омывает платину, раствор€етс€ в ней. Ќасыщенна€ водородом платина начинает вести себ€ как водородный электрод, т.е.
H++к Ђ1/2H2
2H+љH2
jH=0
“.о. за величину электродного потенциала данного электрода принимаетс€ Ёƒ— гальванического элемента, составленного из исследуемого электрода и н.в.э. Ёлектродному потенциалу присваеваетс€ знак, одинаковый со знаком зар€да данного электрода в паре с н.в.э.
Ќапример Zn в паре с н.в.э. зар€жаетс€ отрицательно, а это значит, что потенциал Zn-го электрода равен Ёƒ— Zn-го водородного элемента с обратным знаком.
jZn=j0Zn+RT/n‘*ln aZn2+ jMe=j0Me+RT/n‘*ln[Men+] j0-стандартное значение потенциала.“=298 (250—). jће=j0ће+0.059/n*lg[Men+]
≈сли все металлы выстроить в р€д повелечинам их j0, то мы получим т.н. р€д напр€жений. ¬ р€ду напр€жений помещен и jЌ=0. ¬се металлы, расположенные в р€ду напр€жений выше Ќ имеют j0<0, ниже - j0>0¬ водородном электроде протекает реакци€: H++к ЂH Ц n=1 jH= j0H+0.059/1*lg[H+], j0H =0 по условию. jH =0.059lg[H+], pH=-lg[H+], jH=-0.059pH. потенциала более положительного электрода вычесть потенциал менее положительного. Cu Ц Zn: E=jCu-jZn=j0Cu-j0Zn+RT/n‘*ln([Cu2+]-[Zn2+])=E0 E0-RT/n‘*ln[Zn2+]/[Cu2+] n=2; E0=j0Cu-j0Zn=0.34-(-0.763)=1.103B;E0экспериментальное=1.087¬, DE=0.016B-диффузионный скачок потенциалов на границе 2 растворов.
“ипы электродов и цепей. ќкислительно-восстановительные электроды и цепи.
1. Ёлектроды 1 рода. ћеталлические, обратимые относительно катиона.



2. ћеталлические электроды, обратимые относительно аниона.






3. √азовые электроды, обратимые как относительно катионов, так и анионов.


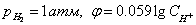


4. ќкислительно-восстановительные электроды.
ћеталлическа€ пластина не принимает участи€, она токоотвод.








“ипы цепей
1. ’имические цепи Ц 2 разных металлических электрода (Al-Zn).
2. ќкислительно-восстановительные цепи (2 ќ¬-электрода)
ќ¬– течет самопроизвольно в сторону превращени€ сильного окислител€ в слабый сопр€женный окислитель, сильного восстановител€ в слабый сопр€женный восстановитель.
3. онцентрационные цепи.

–азные концентрации электролитов.






