¬ода, хот€ и €вл€етс€ слабым электролитом, в небольшой степени диссоциирует:
H2O + H2O ↔ H3O+ + OH−
или
H2O ↔ H+ + OH−
–авновесие этой реакции сильно смещено влево. онстанту диссоциации воды можно вычислить по формуле:
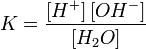 , ,
| (1) |
где:
І [H+] Ч концентраци€ ионов гидроксони€ (протонов);
І [OH−] Ч концентраци€ гидроксид-ионов;
І [H2O] Ч концентраци€ воды (в молекул€рной форме) в воде;
онцентраци€ воды в воде, учитыва€ еЄ малую степень диссоциации, величина практически посто€нна€ и составл€ет (1000 г/л)/(18 г/моль) = 55,56 моль/л.
ѕри 25 ∞C константа диссоциации воды равна 1,8×10−16моль/л. ”равнение (1) можно переписать как:
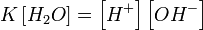 , ,
| (2) |
ќбозначим произведение KЈ[H2O] = Kв = 1,8×10−16 моль/лЈ55,56 моль/л = 10−14моль²/л² = [H+]Ј[OH−] (при 25 ∞C).
онстанта Kв, равна€ произведению концентраций протонов и гидроксид-ионов, называетс€ ионным произведением воды. ќна €вл€етс€ посто€нной не только дл€ чистой воды, но также и дл€ разбавленных водных растворов веществ. C повышением температуры диссоциаци€ воды увеличиваетс€, следовательно, растЄт и Kв, при понижении температуры Ч наоборот.
23. –еакции в растворах электролитов
Ёлектролиты Ц это вещества, растворы которых обладают ионной проводимостью.
ѕоскольку электролиты в растворах образуют ионы, то дл€ отражени€ сущности реакций часто используют так называемые ионные уравнени€ реакций. Ќаписанием ионных уравнений подчЄркиваетс€ тот факт, что, согласно теории диссоциации, в растворах происход€т реакции не между молекулами, а между ионами.
— точки зрени€ теории диссоциации при реакци€х между ионами в растворах электролитов возможны два исхода:
1. ќбразующиес€ вещества Ц сильные электролиты, хорошо растворимые в воде и полностью диссоциирующие на ионы.
2. ќдно (или несколько) из образующихс€ веществ Ц газ, осадок или слабый электролит (хорошо растворимый в воде).
Ќапример, можно рассмотреть две реакции:
2Al + 2NaOH + 6H2O = 2Na[Al(OH)4] + 3H2≠, (1)
2Al + 2KOH + 6H2O = 2K[Al(OH)4] + 3H2≠. (2)
¬ ионной форме уравнени€ (1) и (2) запишутс€ следующим образом:
2Al + 2Na+ + 2OH- + 6 H2O = 2Na+ + 2[Al(OH)4]- + 3H2≠, (3)
2Al + 2K+ + 2OH- + 6 H2O = 2K+ + 2[Al(OH)4]- + 3H2≠, (4)
¬ данном случае алюминий не €вл€етс€ электролитом, а молекула воды записываетс€ в недиссоциированной форме потому, что €вл€етс€ очень слабым электролитом. Ќепол€рные молекулы водорода практически нерастворимы в воде и удал€ютс€ из сферы реакции. ќдинаковые ионы в обеих част€х уравнений (3), (4) можно сократить, и тогда эти уравнени€ преобразуютс€ в одно сокращЄнное ионное уравнение взаимодействи€ алюмини€ с щелочами:
2Al + 2OH- + 6H2O = 2[Al(OH)4]- + 3H2≠. (5)
ќчевидно, что при взаимодействии алюмини€ с любой щелочью реакци€ будет описыватьс€ уравнением (5). —ледовательно, ионное уравнение, в отличие от молекул€рного, относитс€ не к одной какой-нибудь реакции между конкретными веществами, а к целой группе аналогичных реакций. ¬ этом его больша€ практическа€ ценность и значение, например благодар€ этому широко используютс€ качественные реакции на различные ионы.
“ак, при помощи ионов серебра Ag+ можно обнаружить присутствие в растворе ионов галогенов, а при помощи ионов галогенов можно обнаружить ионы серебра; при помощи ионов бари€ Ba2+ можно обнаружить ионы SO2- и наоборот.
— учЄтом вышеизложенного можно сформулировать правило, которым удобно руководствоватьс€ при изучении процессов, протекающих в растворах электролитов.
–еакции между ионами в растворах электролитов идут практически до конца в сторону образовани€ осадков, газов и слабых электролитов.
—ледовательно, реакции идут с образованием веществ с меньшей концентрацией ионов в растворе в соответствии с законом действующих масс. —корость пр€мой реакции пропорциональна произведению концентраций ионов реагирующих компонентов, а скорость обратной реакции пропорциональна произведению концентраций ионов продуктов. Ќо при образовании газов, осадков и слабых электролитов ионы св€зываютс€ (уход€т из раствора) и скорость обратной реакции уменьшаетс€.
|
|
|






