’имическое превращение есть качественный скачок, при котором исчезают одни вещества и образуютс€ другие. ѕроисход€ща€ при этом перестройка электронных структур атомов, ионов и молекул сопровождаетс€ выделением или поглощением тепла, света, электричества и т.п. Ц превращением химической энергии в другой вид энергии.
Ёнергетические эффекты реакций изучает термохими€. ƒанные об энергетических эффектах реакций используютс€ дл€ расчетов тепловых балансов технологических процессов, дл€ определени€ энергии межатомных и межмолекул€рных св€зей, дл€ вы€снени€ строени€ и реакционной способности соединений, дл€ установлени€ направлени€ химических процессов и т.д.
’имические реакции обычно протекают при посто€нном давлении (например, в открытой колбе) или при посто€нном объЄме (например, в автоклаве). ѕроцессы, протекающие при посто€нном давлении, называют изобарными, а при посто€нном объЄме Ц изохорными.
—осто€ние системы описываетс€ с помощью р€да переменных: давлени€, объЄма, температуры, массы, энергии. Ќа основе этих параметров могут быть выведены другие переменные, позвол€ющие характеризовать состо€ние системы и происход€щие в ней изменени€. —реди последних важное значение дл€ химиков имеют внутренн€€ энерги€ U, энтальпи€ H, энтропи€ S, изобарный потенциал G и др.
4.1.1. “ермохими€. «акон √есса
“ермохими€ изучает тепловые эффекты химических процессов. ”равнени€ реакций, в которых учитываютс€ их тепловые эффекты, называют термохимическими. ¬ этих уравнени€х выделение теплоты обозначают знаком (+), а поглощение Ц (Ц), например:
H2(г) + —l2(г) = 2H—l(г) + 183,6 кƒж или
1/2H2 (г)+ 1/2—l2(г)= HCl (г) + 91,8 кƒж∙моль-1
N2 (г)+ ќ2(г)= 2Nќ(г) Ц 180,4 кƒж или
1/2N2 (г)+ ќ2(г)= Nќ(г) Ц 90,2 кƒж∙моль-1
¬ термодинамике прин€та обратна€ система знаков, и выделение теплоты в результате химической реакции обозначают знаком (-), а поглощение теплоты- знаком (+).
“еплотой образовани€ называют теплоту, котора€ поглощаетс€ или выдел€етс€ при образовании химического соединени€ количеством вещества 1 моль из простых веществ при заданных услови€х; теплотой разложени€ Ц теплоту, котора€ поглощаетс€ или выдел€етс€ при разложении химического соединени€ количеством вещества 1 моль на простые вещества; теплотой сгорани€ - теплоту, котора€ выдел€етс€ при сгорании вещества 1 моль.
—огласно закону Ћавуазье-Ћапласа теплота разложени€ сложного вещества равна теплоте его образовани€ из простых веществ, что €вл€етс€ частным случаем закона сохранени€ энергии ΔU
¬ качестве стандартных условий в термодинамике принимаетс€ температура 2 0—. (298 ) и давление 1,013∙105 ѕа. “еплоты образовани€ в этих услови€х называют стандартными, дл€ многих веществ они привод€тс€ в таблицах справочной литературы.
“епловые эффекты химических реакций св€заны с изменением внутренней энергии системы при переходе от исходных веществ к продуктам реакции.
¬нутренн€€ энерги€ (U) Ц это весь запас энергии системы, кроме потенциальной энергии еЄ положени€ и кинетической энергии всей системы в целом. “аким образом, U слагаетс€ из поступательного и вращательного движений молекул, колебательного движени€ атомов и атомных групп в молекуле, движени€ электронов в атомах, €дерной энергии и т.д.
|
|
|
≈сли сиcтема поглощает из внешней среды теплоту Q, то внутренн€€ энерги€ U1 увеличиваетс€ на эту величину за вычетом той еЄ части, котора€ расходуетс€ на работу, совершаемую системой (ј), т.е. U2 =U1 +Q-A или ΔU =Q-A, где ΔU= U2 -U1.
≈сли в ходе процесса объЄм системы остаетс€ посто€нным, т.е. работа расширени€ системы не совершаетс€, то изменение внутренней энергии равно теплоте, поглощенной системой в услови€х посто€нного объЄма: ΔU =Qv. »ндексом V обозначают посто€нство объЄма. »зменение внутренней энергии ΔU, а следовательно, и Qv не зависит от пути процесса, что вытекает из закона сохранени€ энергии.
¬ химической практике чаще используют процессы, протекающие при посто€нном давлении, когда объЄм системы может увеличитьс€ на ΔV, в результате она совершит работу, равную рΔV. ¬ этом случае процесс характеризуетс€ энтальпией- величиной, котора€ определ€етс€ уравнением Ќ=U+pΔV. »зменение энтальпии Δ Ќ= ΔU+pΔV.
ѕри стандартных услови€х ΔЌ обозначают символом ΔЌ0298.ƒл€ экзотермических реакций ΔЌ имеет отрицательные, а дл€ эндотермических Цположительные значени€.
≈сли в ходе процесса при посто€нном давлении системой совершаетс€ только работа расширени€ (ј=рΔV), то ΔU= ΔЌ ЦpΔV или ΔU= ΔЌ Ц A, а так как ΔU=Q Ц A, то в этих услови€х ΔЌ=Qp (индекс ФpФ указывает посто€нство давлени€). “ак как ΔЌ не зависит от пути процесса, то этим свойством обладает и Qp.
≈сли реакци€ протекает в конденсированной системе, т.е. с участием только жидких и твердых веществ, то изменение объЄма ΔV практически равно нулю. “огда pΔV=0, и следовательно, ΔЌ=ΔV. ¬ этом случае тепловые эффекты реакций при посто€нном давлении и посто€нном объЄме практически равны между собой: Qv =Qр.
ѕоэтому при термохимических расчетах дл€ конденсированных систем ограничивающие услови€ р= const или V= const опускаютс€.
ќсновным законом термохимии €вл€етс€ закон √есса (1840): тепловой эффект химических реакций, протекающих при посто€нном объЄме или при посто€нном давлении, не зависит от числа промежуточных стадий и определ€етс€ только начальным и конечным состо€нием системы.
«акон √есса можно иллюстрировать схемой образовани€ —ќ2: ΔЌ=ΔЌ1+ΔЌ2,
— ΔЌ —ќ2
 |
ΔЌ1 ΔЌ2
—ќ
т.е. тепловой эффект реакции равен сумме тепловых эффектов отдельных стадий. »з закона √есса следует, что теплота образовани€ вещества не зависит от способов его получени€.
—ледствие закона √есса: стандартный тепловой эффект реакции равен сумме стандартных теплот образовани€ продуктов реакции за вычетом суммы стандартных теплот образовани€ исходных веществ:
ΔЌ0298(реакции)=∑ ΔЌ0298(прод.) -∑ΔЌ0298(исх.).
ѕри термохимических расчетах энтальпии образовани€ простых веществ (Ќ2, —l2 и др.) в стандартном состо€нии принимаютс€ равными нулю. ѕоэтому тепловой эффект реакции синтеза соединений из простых веществ равен стандартной теплоте их образовани€. Ќапример, дл€ реакции
Ќ2(г)+ ¬r2(г) → 2H¬r(г) ΔЌ0298=2(-36,3)- (0+0)= -72,6 кƒж,
|
|
|
т.е. тепловой эффект равен удвоенной стандартной теплоте образовани€ Ќ¬r и реакци€ €вл€етс€ экзотермической.
«акон √есса позвол€ет также рассчитывать теплоты образовани€ нестабильных соединений и тепловые эффекты реакций, которые нельз€ осуществить экспериментально. “ак, невозможно определить тепловой эффект реакции горени€ графита до оксида —ќ, т.к. при этом всегда образуетс€ то или иное количество —ќ2. ќднако тепловой эффект этой реакции может быть найден по экспериментально определенным теплотам сгорани€ графита до —ќ2 и сгорани€ CO до —ќ2:
— + ќ2 → —ќ2 ΔЌ =-393,5 кƒж/моль
—ќ+ 1/2ќ2→ Cќ2 ΔЌ1 =-283,0 кƒж/моль
—+ 1/2ќ2→ —ќ ΔЌ2 =?
—огласно закону √есса, ΔЌ= ΔЌ1 +ΔЌ2, откуда ΔЌ2 = ΔЌ - ΔЌ1 = - 393,5 Ц (-283,0) = -110,5 кƒж/моль.
Ёнтропи€
Ѕольшинство процессов представл€ет собой два одновременно происход€щих €влени€: передачу энергии и изменение в упор€доченности расположени€ частиц друг относительно друга.
„астицам (молекулам, атомам, ионам) присуще стремление к беспор€дочному движению, поэтому система стремитс€ перейти из более упор€доченного состо€ни€ в менее упор€доченное. оличественной мерой беспор€дка €вл€етс€ энтропи€ S. »зменение энтропии ΔS в изолированной системе, переход€щей из состо€ни€ 1 в состо€ние 2, можно определить соотношением
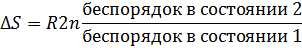
где R- газова€ посто€нна€.
≈сли, например, баллон с газом соединить с вакуумированным сосудом, то газ из баллона будет распредел€тьс€ по всему объЄму сосуда. ѕри этом система из более упор€доченного состо€ни€ (с меньшим беспор€дком) переходит в состо€ние менее упор€доченное (с большим беспор€дком). “аким образом, при переходе системы из более упор€доченного состо€ни€ в менее упор€доченное состо€ние энтропи€ возрастает.
ѕереход же системы из менее упор€доченного состо€ни€ в более упор€доченное состо€ние св€зан с уменьшением энтропии, и самопроизвольное протекание подобного процесса менее веро€тно. “ак, €сно, что в рассматриваемом примере система самопроизвольно не может перейти из состо€ни€ 2 в состо€ние 1, т.е. неверо€тно, чтобы газ сам собой собралс€ в баллоне. ¬ случае перехода системы из менее упор€доченного состо€ни€ в более упор€доченное ΔS системы - величина отрицательна€, т.е. энтропи€ S системы уменьшаетс€.
Ёнтропи€ возрастает при переходе жидкости в пар, при растворении кристаллического вещества и т.д. ¬ процессах конденсации и кристаллизации вещества энтропи€ уменьшаетс€. Ёнтропи€ вещества в газовом состо€нии значительно больше, чем в жидком, а тем более, чем в твердом.
Ёнтропии веществ, как и их теплоты образовани€, прин€то относить к определенным услови€м, обычно при температуре 25 0— (298 ) и давлении 1 атм. Ёнтропию при этих услови€х обозначают S0298 и называют стандартной энтропией.
«начени€ми энтропии веществ пользуютс€ дл€ установлени€ изменени€ энтропии системы в результате соответствующих процессов. “ак, дл€ химической реакции
ај+bB+Е= dD+е≈+Еизменение энтропии системы будет
ΔS= (dSD + eSE+Е)-(aSA + bSB+...) или ΔS= ∑Sпрод. - ∑Sисх.
ќб изменении энтропии в химических реакци€х можно судить по изменению объЄма системы в ходе реакции. Ќапример, в реакции ½ — (графит)+½ —ќ2(г)=—ќ(г) наблюдаетс€ увеличение объЄма ΔV>0; следовательно, энтропи€ возрастает ΔS> 0 (ΔS0298=87,7 дж/моль∙град). ¬ случае же реакции образовани€ Ќ3N из водорода и азота 3/2 Ќ2 (г)+1/2 N2 (г)= H3N(г) наоборот, объЄм системы уменьшаетс€ ΔV<0; следовательно, энтропи€ уменьшаетс€ ΔS<0 (ΔS0298=99,1дж/моль∙град).
≈сли же реакци€ протекает между твердыми веществами, например,
јl(к)+Sb(к)=јlSb(к), то изменени€ объЄма системы и еЄ энтропии практически не происходит (ΔS0298=4,03дж/моль∙град). “о же самое относитс€ и к процессам, в которых число молей газообразных веществ не измен€етс€, например, — (графит)+ ќ2 (г)= —ќ2 (г), ΔS=2,9дж/моль∙град.
|
|
|
4.1.3. Ёнерги€ √иббса и направленность химических процессов
¬озможность самопроизвольного протекани€ химического процесса определ€етс€ двум€ факторами: стремлением системы к понижению внутренней энергии за счет экзотермической реакции (-∆H) и стремлением системы к увеличению неупор€доченности в расположении частиц за счет теплового движени€, мерой которой €вл€етс€ функци€ состо€ни€, называема€ энтропией S.
≈сли ∆H незначительно зависит от температуры, то энтропи€ с повышением температуры сильно возрастает. ¬ли€ние как энтальпийного так и энтропийного факторов учитываетс€ в уравнении ∆G=∆H-T∆S. ‘ункцию G называют энергией √иббса, она €вл€етс€ мерой устойчивости системы в услови€х T=const и p=const.
—осто€ние системы при посто€нном объеме описываетс€ функцией, которую называют энергией √ельмгольца: ∆P=∆U-T∆S. “аким образом, ∆G отличаетс€ от ∆P так же, как ∆H от ∆U на величину, равную работе расширени€ p∆V.
»зменение энергии √иббса ∆G определ€ет возможность или невозможность самопроизвольного протекани€ процесса. ≈сли ∆G<0, т.е. по ходу реакции происходит уменьшение энергии √иббса, то этот процесс термодинамически возможен. ≈сли ∆G>0, т.е. процесс ведет к увеличению энергии √иббса, то така€ реакци€ термодинамически невозможна. ≈сли ∆G=0, то реакционна€ система находитс€ в состо€нии равновеси€.
ѕри положительном значении ∆S и, следовательно, T∆S величина G будет отрицательной: а) при любых отрицательных значени€х Ќ, т.е. все экзотермические процессы в этом случае возможны; б) при положительных значени€х ∆Ќ, но при условии, что по абсолютному значению ∆Ќ<T∆S, т.е. эндотермические реакции возможны, когда энтропийный фактор преобладает над энтальпийным, что легче реализуетс€ с ростом температуры.
ѕри отрицательном значении ∆S в уравнении ∆G=∆H-T∆S величина T∆S становитс€ положительной, поэтому в этом случае эндотермические реакции (+∆Ќ) самосто€тельно протекать не могут, так как ∆G будет величиной также положительной. »з экзотермических реакций (-∆Ќ) при таком условии возможны только те, которые характеризуютс€ большим отрицательным значением ∆Ќ, т.е. те эндотермические реакции, дл€ которых сумма -∆Ќ+(-“∆S) имеет отрицательное значение.
ѕри очень низких температурах величина “∆S минимальна, так как и энтропи€ в этих услови€х измен€етс€ незначительно, поэтому преимущественное вли€ние на направление процесса имеет энтальпийный фактор и обычно процессы идут в сторону экзотермических реакций. ¬ конденсированных системах реакции протекают с незначительным изменением энтропии, поэтому знак ∆G определ€етс€ знаком ∆Ќ.
“аким образом, дл€ определени€ возможности протекани€ процесса при данных услови€х надо найти знак ∆G. — этой целью в справочных таблицах наход€т стандартные значени€ ∆Ќ и S дл€ веществ, участвующих в реакции, по которым вычисл€ют ∆G на формулы ∆Go298=∆H o298-T∆S o298 [3].
ѕример 1. –асчет теплового эффекта химической реакции по теплоте образовани€ реагирующих веществ и продуктов реакции.
ќпределите количество теплоты, выдел€ющейс€ при гашении 100кг извести водой при 25о—, если известны стандартные теплоты образовани€ веществ, участвующих в химической реакции:
∆H o298, CaO(к) = -635,1 кƒж∙моль-1;
∆H o298, H2O(ж) = -285,84 кƒж∙моль-1;
∆H o298, Ca(OЌ)2(к) = -986,2 кƒж∙моль-1.
–ешение. –еакци€ гашени€ извести: —аќ(к)+Ќ2ќ(ж)=—а(ќЌ)2(к). —огласно первому следствию из закона √есса тепловой эффект химической реакции равен разности между суммой теплот образовани€ реагирующих веществ с учетом стехиометрических коэффициентов:
|
|
|
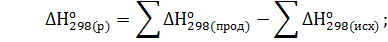
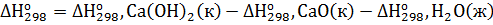 =
=
=-986,2-(-635,1)-(-285,84) = 65,26 кƒж∙моль-1
“аким образом, при гашении водой 1 моль извести выдел€етс€ 65,26кƒж, при гашении 1кмоль извести Ц 65260 кƒж. ћ(—аќ) = 56г∙моль-1=56кг∙моль-1; m(—аќ) = 100кг;
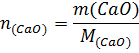
ѕри гашении водой 100 кг извести выдел€етс€ 100/56∙65260=116536кƒж.
ѕример 2. –асчет теплоты образовани€.
ќпределить теплоту образовани€ —а(ќЌ)2тв на основе реакций:
—а—ќ3→—аќ+—ќ2+∆Ќ1; —аќ+Ќ2ќ→—а(ќЌ)2тв+∆Ќ2.
–ешение. ƒл€ определени€ суммарной теплоты образовани€ ∆Ќ дл€ этой реакции определ€ем ∆Ќ1 и ∆Ќ2, тогда ∆Ќ=∆Ќ1+∆Ќ2;
∆Ќ1=∆Ќ—аќ+∆Ќ(—ќ2)-∆Ќ—а—ќ3 = +177,83 кƒж∙моль-1;
∆Ќ2=∆Ќ—а(ќЌ)2-∆Ќ—аќ-∆ЌЌ2ќ = -65,2 кƒж∙моль-1;
∆Ќ= +177,83 Ц 65,2 = +113,61 кƒж∙моль-1.
ѕример 3. –ассчитайте тепловой эффект реакции горени€ сероводорода по следующим данным: Ќ2S(г) + 3/2O2=Ќ2ќ(ж) + Sќ2(г)
1)S(к) + Ќ2 = Ќ2S(г); ∆Ќ1= -20,17 кƒж
2)Ќ2 + 1/2 O2 = H2O(ж); ∆Ќ2= -286,0 кƒж
3)S(к) + ќ2 = Sќ2(г); ∆Ќ3= -297 кƒж
–ешение. ”равнение 1-3 есть термохимические уравнени€ образовани€ соответственно 1 моль Ќ2S(г), Ќ2ќ(ж), Sќ2(г) из простых веществ в стандартных услови€х: “=298 и –=101,325 кѕа, а тепловые эффекты Ц стандартные энтальпии образовани€ указанных соединений ∆H o298. ≈сли сложить термохимические уравнени€ 2 и 3 и вычесть уравнение 1, получим искомое уравнение
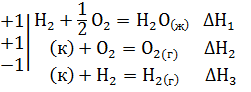
Ќ2S(г) + 3/2 O2 = H2O(ж) + SO2(г) ∆Ќ=∆Ќ1+∆Ќ2-∆Ќ3
ѕодставить численное значение энтальпий образовани€ Ќ2S(г) , Ќ2ќ(ж), SO2(г), получим значение теплового эффекта реакции ∆Ќ= -286 -297 Ц(-20,17) = = -562,8 кƒж. ќтрицательное значение энтальпии реакции горени€ сероводорода означает, что данна€ реакци€ экзотермическа€.
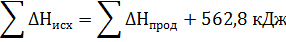
“епловой эффект можно включить в уравнение химической реакции.
Ќ2S(г) + 3/2ќ2 = Ќ2ќ(ж) + SO2(г) + 562,8 кƒж. Ёта запись также представл€ет собой термохимическое уравнение реакции.
ѕример 4. ќпределение изменени€ энтропии в стандартных услови€х. ќпределить изменение энтропии реакции Na2O+SiO2=Na2SiO3 при температуре 298 .
–ешение. Ќаходим по справочнику значени€ энтропии продукта и исходных веществ при “=298 , S o298, Na2O(т)=42,09ƒж∙моль-1∙к-1:
S o298, SiO2 (т) = 71,1ƒж∙моль-1∙к-1;
S o298, Na2SiO3 (т)=113,8ƒж∙моль-1∙к-1. »зменение энтропии в химической реакции равно разности между суммой энтропий продуктов реакции и суммой энтропий исходных веществ с учетом стехиометрических коэффициентов.
So298 х.р. = So298, Na2SiO3(т) Ц So298, SiO2 (т) - So298, Na2O(т) = 113,8-71,1-42,09=0,61 ƒж∙моль-1∙к-1
ѕример 5. ќпределение изменени€ энтропии при фазовых превращени€х. ќпределить изменение энтропии 1г кристобалита при температуре плавлени€ 1986 , если теплота обратного фазового превращени€ кристобалита равна 744,05 ƒж∙r-1.
–ешение. »зменение энтропии при переходе вещества из одного агрегатного состо€ни€ в другое равно: 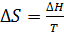 , где ∆Ќ-теплота обратимого фазового превращени€.
, где ∆Ќ-теплота обратимого фазового превращени€.
S2-S1=∆S= 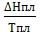 =
= 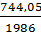 = 0,238ƒж∙г-1∙град-1.
= 0,238ƒж∙г-1∙град-1.
ѕример 6. ¬ычислите 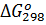 дл€ химической реакции 2SO2(г)+ќ2(г)↔2SO3(г). ¬ каком направлении может протекать эта реакци€ в стандартных услови€х?
дл€ химической реакции 2SO2(г)+ќ2(г)↔2SO3(г). ¬ каком направлении может протекать эта реакци€ в стандартных услови€х?
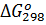 , SO2(г) = -300,37 кƒж∙моль-1;
, SO2(г) = -300,37 кƒж∙моль-1;
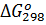 , SO3(г) = -370,37 кƒж∙моль-1
, SO3(г) = -370,37 кƒж∙моль-1
–ешение. »зменение энергии √иббса в химической реакции
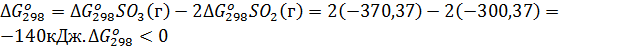 , поэтому в стандартных услови€х данна€ реакци€ может протекать в сторону образовани€ SO3.
, поэтому в стандартных услови€х данна€ реакци€ может протекать в сторону образовани€ SO3.
’имическа€ кинетика
ѕример 1. ѕри взаимодействии кристаллов хлорида фосфора (V) с парами воды образуетс€ жидкий –ќ—13 и хлороводород. –еакци€ сопровождаетс€ выделением 111,4 кƒж теплоты. Ќапишите термохимическое уравнение этой реакции.
–ешение. ”равнени€ реакций, в которых около символов химических соединений указываютс€ их агрегатные состо€ни€ или кристаллическа€ модификаци€, а также числовое значение тепло≠вых эффектов, называют термохимическими. ¬ термохимических уравнени€х, если это специально не оговорено, указываютс€ значени€ тепловых эффектов при посто€нном давлении Qp, равные изменению энтальпии системы ∆Ќ. «начение ∆Ќ привод€т обычно в правой части уравнени€, отдел€€ его зап€той или точкой с зап€той. ѕрин€ты следующие сокращенные обозначени€ агрегатного состо€ни€ вещества: г Ч газообразное, ж Ч жидкое, к Ч крис≠таллическое. Ёти символы опускаютс€, если агрегатное состо€ние веществ очевидно.
≈сли в результате реакции выдел€етс€ теплота, то ∆Ќ < 0. —читыва€ сказанное, составл€ем термохимическое уравнение данной в примере реакции:
|
|
|
–—15(к) + Ќ2ќ(г) = –ќ—l 3(ж) + 2Ќ—1(г); ∆Ќхр = -111,4 кƒж
“аблица 3
—тандартные теплоты (энтальпии) образовани€ 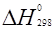 некоторых веществ
некоторых веществ
| ¬ещество | —осто- €ние | ∆Ќ 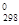 , кƒж/моль , кƒж/моль
| ¬ещество | —осто-€ние | ∆Ќ 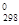 , кƒж/моль , кƒж/моль
|
| —2Ќ2 —S2 Nќ —6Ќ6 —2Ќ4 Ќ2S NЌ3 —Ќ4 —2Ќ6 Ќ—l | г г г г г г г г г г | +226,75 +115,28 +90,37 +82,93 +52,28 -20,15 -46,19 -74,85 -84,62 -92,31 | —ќ —Ќ3ќЌ —2Ќ5ќЌ Ќ2ќ Ќ2ќ NЌ4—l —ќ2 Fе2ќ3 “iќ2 —а(ќЌ)2 јl2ќ3 | г г г г ж к г к к к к | -110,52 -201,17 -235,31 -241,83 -285,84 -315,39 -393,51 -822,10 -943,90 -986,50 -1669,80 |
ѕример 2. –еакци€ горени€ этана выражаетс€ термохимическим уравнением —2Ќ6(г) + 3 ½ ќ2 = 2 —ќ2(г) + 3 Ќ2ќ(ж); Ќхр = -1559,87 кƒж.
¬ычислите теплоту образовани€ этана, если известны теплоты образовани€ —ќ2(г) и Ќ2ќ(ж) (см. табл. 5).
–ешение. “еплотой образовани€ (энтальпией) данного соединени€ называют тепловой эффект реакции образовани€ 1 моль этого соединени€ из простых веществ, вз€тых в их устойчивом состо€нии при данных услови€х. ќбычно теплоту образовани€ относ€т к стандартному состо€нию, т.е. 25 о— (298 ) и 1,013∙105 ѕа и обозначают через ∆Ќ 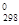 . “ак как тепловой эффект с температурой измен€етс€ незначительно, то в дальнейшем индексы опускаютс€ и тепловой эффект обозначаетс€ через ∆Ќ. —ледовательно, нужно вычислить тепловой эффект реакции, термохимическое уравнение которой имеет вид
. “ак как тепловой эффект с температурой измен€етс€ незначительно, то в дальнейшем индексы опускаютс€ и тепловой эффект обозначаетс€ через ∆Ќ. —ледовательно, нужно вычислить тепловой эффект реакции, термохимическое уравнение которой имеет вид
2— (графит) + «Ќ2(г) - —2Ќ6(г); ∆Ќ=?
исход€ из следующих данных:
а) —2Ќ6(г) + 3'/2ќ2(г) = 2—ќ2(г) + «Ќ2ќ(ж); ∆Ќ= -1559,87 кƒж;
б) — (графит) + ќ2(г) = —ќ2(г); ∆Ќ = -393,51 кƒж;
в) Ќ2(г) + ½ O2 = Ќ2ќ(ж); ∆Ќ = - 285,84 кƒж.
Ќа основании закона √есса с термохимическими уравнени€ми можно оперировать так же, как и с алгебраическими. ƒл€ получени€ искомого результата следует уравнение (б) умножить на 2, уравнение (в) Ч на 3, а затем сумму этих уравнений вычислить из уравнени€ (а):
—2Ќ6 + 372ќ2 - 2C - 2ќ2-«Ќ2 -3/2ќ2 = 2—ќ2 + «Ќ2ќ -2—ќ2 - «Ќ2ќ
∆Ќ = -1559,87 -2(-393,51)-3(-285,84) = +84,67 кƒж;
∆Ќ = -1559,87 + 787,02 + 857,52; —2Ќ6 = 2— + «Ќ2; ∆Ќ= + 84,67 кƒж.
“ак как теплота образовани€ равна теплоте разложени€ с обратным знаком, то ∆Ќ 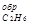 (г) = -84,67 кƒж. тому же результату придем, если дл€ решени€ задачи применить вывод из закона √есса:
(г) = -84,67 кƒж. тому же результату придем, если дл€ решени€ задачи применить вывод из закона √есса:
∆Ќх р = 2∆Ќ—ќ2 + «∆ЌЌ2ќ - ∆Ќ—2н6 - 3 ½ ∆Ќќ2
”читыва€, что теплоты образовани€ простых веществ условно прин€ты равными нулю ∆Ќс2н6 = 2∆Ќ—ќ  + «∆ЌЌ
+ «∆ЌЌ  ќ - ∆Ќх р
ќ - ∆Ќх р
∆Ќс2н6 = 2(-393,51) + 3(-285,84) + 1559,87 = -84,67;
то ∆Ќ 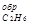 (г) = -84,67 кƒж
(г) = -84,67 кƒж
ѕример 3. –еакци€ горени€ этилового спирта выражаетс€ термохимическим уравнением
—2Ќ5ќЌ(ж) + «ќ2(г) = 2—ќ2(г) + «Ќ2ќ(ж); ∆Ќ=?
¬ычислите тепловой эффект реакции, если известно, что мол€рна€ теплота парообразовани€ —2Ќ5ќЌ(ж) равна +42,36 кƒж, а теплоты образовани€ —2Ќ5ќЌ(г), —ќ2(г), Ќ2ќ(ж) см. табл. 5.
–ешение. ƒл€ определени€ ∆Ќ реакции необходимо знать теплоту образовани€ —2Ќ5ќЌ(ж). ѕоследнюю находим из данных:
—2Ќ5ќЌ(ж) = —2Ќ5ќЌ(г); ∆Ќ= +42,36 кƒж +42,36=-235,31-∆Ќ—2Ќ5ќЌ (ж);
∆Ќ—2Ќ5ќЌ (ж) = -235,31 - 42,36 = -277,67 кƒж.
¬ычисл€ем реакции, примен€€ следстви€ из закона √есса:
∆Ќхр = 2(-393,51) + 3(-285,84) + 277,67 = -1366,87 кƒж.
ѕример 4. ¬ каком состо€нии энтропи€ 1 моль вещества больше при одинаковой температуре: в кристаллическом или парообразном?
–ешение. Ёнтропи€ есть мера неупор€доченности состо€ни€ вещества. ¬ кристалле частицы (атомы, ионы) расположены упор€доченно и могут находитьс€ лишь в определенных точках пространства, а дл€ газа таких ограничений нет. ќбъем 1 моль газа гораздо больше объема 1 моль кристаллического вещества; возможность хаотичного движени€ молекул газа больше. ј так как энтропию можно рассматривать как количественную меру хаотичности атомно-молекул€рной структуры вещества, то энтропи€ 1 моль паров вещества больше энтропии 1 моль его кристаллов при одинаковой температуре.
ѕример 5. ѕр€ма€ или обратна€ реакци€ будет протекать при стандартных услови€х в системе —Ќ4(г) + —ќ2  2—ќ(г) + 2Ќ2(г)
2—ќ(г) + 2Ќ2(г)
–ешение. ¬ычислим ∆G0298 пр€мой реакции. «начени€ ∆G0298 соответствующих веществ приведены в таблице 4. «на€, что ∆G есть функци€ состо€ни€ и что ∆G дл€ простых веществ, наход€щихс€ в устойчивых при стандартных услови€х агрегатных состо€ни€х, равны нулю, находим ∆G0298 процесса: ∆G0298 = 2(-137,27) + 2(0) - (-50,79 - 394,38) = + 170,63 кƒж.
“о что ∆G0298 > 0, указывает на невозможность самопроиз≠вольного протекани€ пр€мой реакции при “ = 298 и давлении вз€тых газов, равном 1,013 ∙105 ѕа (760 мм рт. ст. = 1 атм).
“аблица 4
—тандартна€ энерги€ √иббса образовани€ 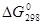 некоторых веществ
некоторых веществ
| ¬ещество | —осто€ние | 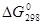 ,
кƒж/моль ,
кƒж/моль
| ¬ещество | —осто€ние | 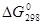 кƒж/моль
кƒж/моль
|
| ¬аSќ4 | -1138,8 | FeO | -244,3 | ||
| —а—ќ3 | -1128,75 | H2O | ∆ | -237,19 | |
| Fе3ќ4 | -1014,2 | H2O | √ | -228,59 | |
| ¬е—ќ3 | -944,75 | PbO2 | -219,0 | ||
| —аќ | -604,2 | CO | √ | -137,27 | |
| ¬еќ | -581,61 | CH4 | √ | -50,79 | |
| NаF | -541,0 | NO2 | √ | +51,84 | |
| ¬аќ | -528,4 | NO | √ | +86,69 | |
| —ќ2 | √ | -394,38 | C2H2 | √ | +209,20 |
| NaCl | -384,03 | ||||
| ZnO | -318,2 |
“аблица 5
—тандартные абсолютные энтропии 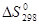 некоторых веществ
некоторых веществ
| ¬ещество | —осто€ ние | 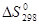 ƒж/
(моль∙ ) ƒж/
(моль∙ )
| ¬ещество | —осто€ ние | 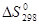 ƒж/(моль∙ ) ƒж/(моль∙ )
|
| — | алмаз | 2.44 | H2O | √ | 188,72 |
| — | √рафит | 5.69 | N2 | √ | 191,49 |
| Fe | 27.2 | NH3 | √ | 192,50 | |
| Ti | 30.7 | CO | √ | 197,91 | |
| S | –омб | 31.9 | C2H2 | √ | 200,82 |
| TiO2 | 50.3 | O2 | √ | 205,03 | |
| FeO | 54.0 | H2S | √ | 205,64 | |
| H2O | ∆ | 69.94 | NO | √ | 210,20 |
| Fe2O3 | 89.96 | CO2 | √ | 213,65 | |
| NH4Cl | 94.5 | C2H4 | √ | 219,45 | |
| CH3OH | ∆ | 126.8 | Cl2 | √ | 222,95 |
| H2 | √ | 130.59 | NO2 | √ | 240,46 |
| Fe3O4 | 146.4 | PCl3 | √ | 311,66 | |
| CH4 | √ | 186.19 | PCl5 | √ | 352,71 |
| HCl | √ | 186.69 |
ѕример 6. Ќа основании стандартных теплот образовани€ (см. табл. 3) и абсолютных стандартных энтропии веществ (табл. 5) вычислите ∆S0298 реакции, протекающей по уравнению —ќ(г) + Ќ2ќ(ж) = —ќ2(г) + Ќ2(г)
–ешение. ∆G0 = ∆Ќ - “∆S0; ∆Ќ и ∆S Ч функции состо€ни€, поэтому
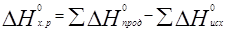
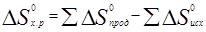
∆Ќ 0х.р. = (-393,51+0) Ц (-110,52-285,84) = +2,85 кƒж
∆S 0х.р. =(213.65 + 130.59) Ц (197.91 + 69.94) = +79.39 = 0.07639кƒж/(моль∙ )
∆G0 = +2,85 Ц 298 ∙ 0,07639 = - 19,91 кƒж.
ѕример 7. –еакци€ восстановлени€ Fe2O3 водородом протекает по уравнению Fe2O3(к)+ 3H2(г) = 2Fe(к) + «Ќ2ќ(г); ∆Ќ= +96,61 кƒж
¬озможна ли эта реакци€ при стандартных услови€х, если изменение энтропии ∆S = 0,1387 кƒж/(моль∙ )? ѕри какой температуре начнетс€ восстановление Fe2ќ3?
–ешение. ¬ычисл€ем ∆G0 реакции: ∆G =∆H-T∆S = 96,61 -298 ∙ 0,1387 = +55,28 кƒж.
“ак как ∆G > 0, то реакци€ при стандартных услови€х невоз≠можна; наоборот, при этих услови€х идет обратна€ реакци€ окислени€ железа (коррози€). Ќайдем температуру, при которой ∆G =0:
∆Ќ = “∆S; 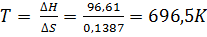
—ледовательно, при температуре ≈ 696,5 начнетс€ реакци€ восстановлени€ Fe2O3. »ногда эту температуру называют температурой начала реакции.
ѕример 8. ¬ычислите ∆Ќ∞, ∆S и ∆G0т реакции, протекающей по уравнению Fe2O3(к) + «— = 2Fe + «—ќ. ¬озможна ли реакци€ восстановлени€ Fe2O3 углеродом при 500 и 1000 ?
–ешение. ∆Ќ 0х.р. и ∆S 0х.р. находим из соотношений (1) и (2):
∆Ќ 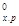 = [3(-110.52)+2∙0]-[-822.10+3∙0]
= [3(-110.52)+2∙0]-[-822.10+3∙0]
Ёнергию √иббса при соответствующих температурах находим из соотношени€
∆G500= 490,54 -500 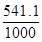 = +219,99кƒж
= +219,99кƒж
∆G1000 = 490,54 - 1000 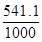 = -50.56кƒж
= -50.56кƒж
“ак как ∆G500 >0, а ∆G1000 <0, то восстановление Fe2O3 возможно при 1000 и невозможно при 500 .






