Ёлектроды
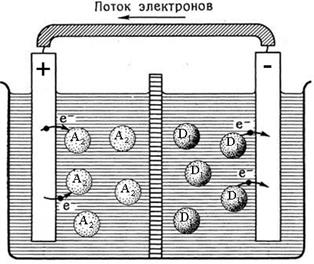
ѕерераспределение электронной плотности в окислительно-восстанови-тельной реакции D1 + A2 Ѓ A1 + D2, если проводить ее в специальном устройстве - гальваническом элементе, может быть использовано дл€ создани€ электродвижущей силы (Ёƒ—). –ис. 5.1 иллюстрирует, каким образом химическую энергию можно превратить в электрическую.

| 
|
–ис. 5.1. —лева Ц хаотические переходы электронов в массе окислительно-восстанови-тельной реакции; справа Ц упор€доченные электронные переходы в гальваническом элементе.
√альванический элемент образован электродами (основна€ его часть) и может быть дополнен мембраной, проницаемой дл€ одного типа ионов.
–ассмотрим простейший тип электрода металл - ион металла Mz+│M, соответствующий обратимой окислительно-восстановительной полуреакции между ионами металла из раствора и электронами из электрода с образованием нейтральных атомов электрода. Ёта гетерогенна€ реакци€ протекает на границе раздела фазы раствора (S Ц solution) и фазы металлического (ћ - metal) электрода
M z + (S) + z eЦ ЂM (M); M z +/ z + eЦ ЂM/ z, (5.1)
z eЦ Ц избыточна€ часть Ђэлектронного газаї в металле. ѕодразумева€ фазовую принадлежность каждого компонента, чаще используют сокращенную форму записи уравнени€ полуреакций (втора€ в (5.1)).
| ¬ зависимости от природы раствора и металла возможны два варианта. 1) јтомы металла при его растворении покидают кристаллическую решетку и переход€т в раствор в виде положительных ионов. «а счет оставленных на электроде электронов он приобретает отрицательный зар€д и, соответственно, отрицательный потенциал, преп€тствующий дальнейшему растворению электрода. 2) »оны осаждаютс€ на электроде. Ќедостаток электронов, которые нейтрализуют приход€щие из раствора ионы, формирует положительный потенциал электрода. | |
| –ис. 5.2. ѕроцессы на электроде. |
–авновесие электрод - раствор и в том, и в другом случае определ€етс€ равенством электрохимических потенциалов i -х компонентов в фазе с потенциалом φ. 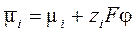 , (5.2)
, (5.2)
где F = 96485.3 л/моль Ц число ‘араде€ (абсолютна€ величина зар€да мол€ электронов). ќтметим, что электрохимический потенциал, по определению, кроме химического потенциала (μ i) включает в себ€ электростатическую энергию мол€ частиц с зар€дом zi в поле с потенциалом φ.
»так, дл€ реакции (5.1) на электроде M z +| M равновесие и соответствующий равновесный потенциал определ€ютс€ равенством
 , (5.3)
, (5.3)
где штрих отмечает подфазу электронного УгазаФ электрода. ѕри подстановке (5.2) в (5.3) получаем
 . (5.4)
. (5.4)
ѕодставл€€ выраженный через активность химпотенциал катиона в растворе  находим межфазную разность потенциалов
находим межфазную разность потенциалов
 (5.5)
(5.5)
ѕри условии отсчета энергии от состо€ни€ простых веществ химический потенциал электрода можно считать равным нулю:  . ¬вед€ обозначение дл€ оставшейс€ части первого слагаемого (5.5)
. ¬вед€ обозначение дл€ оставшейс€ части первого слагаемого (5.5)
 (5.6)
(5.6)
окончательно получаем (штрих, детализирующий фазу ћ, как и далее Ц опущен)
 (5.7)
(5.7)
–азность потенциалов на границе раздела фаз электрод (металл) Ц раствор ионов этого металла (межфазна€ разность потенциалов) выражена через стандартную разность потенциалов (индекс Ђ˚ї) и активность (напомним Ц исправленную концентрацию) ионов в растворе. ¬ свою очередь, стандартное значениеразности потенциалов, выраженное через стандартные химические потенциалы компонентов фаз, как прин€то, соответствует активности равной 1.
|
|
|
—ледующий тип так называемых газовых электродов (рис. 5.3) образован трем€ сосуществующими фазами: исходный раствор, газ, обе эти фазы контактируют с фазой инертного металла. ќбозначение электрода: G± | G2 | Pt (если инертный металл - платина).
¬ариант (а) 1/2 G2 + eЦ ЂGЦ, например GЦ ≡ ClЦ Ц ионы хлора в растворе;
¬ариант (б) G+ + eЦ Ђ1/2 G2, например G+ ≡ H+.

| ”словие равновеси€ дл€ приэлектродной химической реакции (вариант (а)), как и ранее определ€етс€ соответствующим уравнением дл€ электрохимических потенциалов:
 . (5.8) . (5.8)  , ,
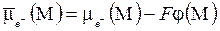 , ,
 . ѕервый из вход€щих в (5.8) электрохимических потенциалов дл€ не зар€женного газового компонента равен химическому потенциалу. . ѕервый из вход€щих в (5.8) электрохимических потенциалов дл€ не зар€женного газового компонента равен химическому потенциалу.
|
| –ис. 5.3. Ёлектрод газ |инертный металл. |
ѕри этом в отличие от идеального химический потенциал неидеального газа, выражаетс€ не через давление, а через летучесть f, аналогично активности соответствующей исправленному давлению.
¬ полученной после аналогичных преобразований разности потенциалов уравнению (а) соответствует верхний знак, уравнению (б) - нижний:
 , (5.9)
, (5.9)
где  Ц стандартное значение межфазного потенциала при f и a = 1.
Ц стандартное значение межфазного потенциала при f и a = 1.

| Ёлектрод - ион | нерастворима€ соль | металл (XЦ | MX | M) имеет две поверхности раздела. — одной стороны соль MX контактирует с раствором, содержащим анионы XЦ, а с другой стороны - с металлом M. ќбычный пример - хлорсеребр€ный электрод: ClЦ | AgCl | Ag. ≈ще один пример такого электрода - так называемый каломельный электрод ClЦ | Hg2Cl2 | Hg. Ёлектродна€ реакци€ MX + e‾ ↔ M + X‾ включает компоненты трех фаз, что учитываетс€ при записи услови€ равновеси€ (5.10): |
| –ис. 5.4. Ёлектрод ион | нерастворима€ соль | металл |
 , (5.10)
, (5.10)
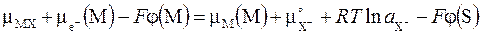 ,
,
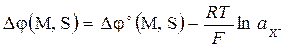 , (5.11)
, (5.11)
где  . (5.12)
. (5.12)
¬се рассмотренные выше электроды - материализаци€ окислительно-восстановительных или редокс-реакций, св€занных с переносом электронов. ќднако термин редокс-электрод примен€етс€ в тех случа€х, когда вещества в растворе существуют в двух окислительных состо€ни€х.
ќбозначение редокс-электрода A2+, A+ | M, где M - инертный металл соответствует полуреакции A2+ + eЦ = A+.
ѕримеры: Fe3+ + eЦ ЂFe2+ - биологически важное гидрохиноновое равновесие.
¬ывод, подобный предыдущим, дает выражение дл€ межфазной разности потенциалов и его стандартной составл€ющей
 , (5.13)
, (5.13)
 . (5.14)
. (5.14)
¬ажно, что положение равновеси€ с необходимым соотношением окисленных форм можно регулировать изменением разности потенциалов. — другой стороны раствор с заданным соотношением концентраций восстановленных и окисленных ионов позвол€ет поддерживать требуемую разность потенциалов.

| ≈сли два раствора соли MX различной концентрации разделить мембраной, проницаемой, например, только дл€ катионов M+, то за счет их диффузии в разбавленный раствор устанавливаетс€ мембранный потенциал:
 Ц следствие равенства электрохимических потенциалов фаз α и β с различной концентрацией ионов.
ќдин из "живых" примеров мембранной системы - стенка биологической клетки,
Ц следствие равенства электрохимических потенциалов фаз α и β с различной концентрацией ионов.
ќдин из "живых" примеров мембранной системы - стенка биологической клетки,
|
| –ис. 5.5. ¬озникновение мембранного потенциала. |
котора€ более проницаема дл€ ионов K+, чем дл€ Na+ и Cl¯. ¬нутри клетки концентраци€ K+ в 10‑30 раз больше, чем снаружи и поддерживаетс€ действием "специального насоса". √орючее дл€ него Цаденозинтрифосфорна€ кислота (ј“‘). ”правление ферментами. Dj(in,ex) = 0,07 ¬.
|
|
|
»мпульс 0,02 ¬ измен€ет структуру мембраны, и она становитс€ проницаемой дл€ Na+. Ёто приводит к уменьшению Dj(in,ex), которое в свою очередь приводит в активность соседние клетки и т.д., что формирует волну передачи нервных импульсов.
√альванические элементы
омбинаци€ двух электродов образует гальванический элемент или гальваническую €чейку.

| 
|
| –ис. 5.6. √альванический элемент без жидкостного соединени€. | –ис. 5.7 а. ѕроточный вариант жидкостного соединени€. |
 –ис. 5.7 б.—олевой мостик.
–ис. 5.7 б.—олевой мостик.
|
Ќапример, сочетание водородного и хлорсеребр€ного электродов дает наиболее простой элемент без жидкостного соединени€, т.е. элемент с общим электролитом (рис. 5.6).
Pt, H2 | HCl | AgCl, Ag, Pt
≈сли два электрода, левый (L) и правый (R), помещены в два разных электролита, то образуетс€ элемент с жидкостным соединением.
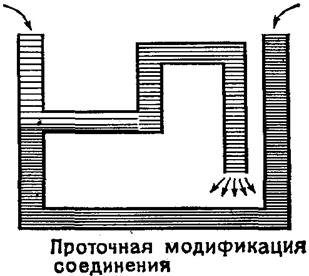
¬арианты жидкостного соединени€ дл€ так называемого концентрационного элемента (электролиты у левого и правого электродов отличаютс€ только концентрацией, в данном случае в единицах мол€льности) показаны на рис. 5.7.
Pt, H2 | HCl(m L) | HCl(m R) |H2, Ag, Pt
ƒругим способом жидкостного соединени€ полуэлементов €вл€етс€ так называемый солевой мостик (рис. 5.7), приготовленный растворением KCl в водорастворимом желе. “ок в мостике определ€етс€ высокой концентрацией переносимых ионов K+ и Cl¯. —читаетс€, что дополнительные потенциалы "солевой мостик" ‑ раствор взаимно сокращаютс€. ѕричины и механизм успешного действи€ солевого мостика к насто€щему времени до конца не вы€снены.
ƒиффузионные контакты двух растворов посредством жидкостного соединени€ внос€т элементы неравновесности, осложн€ющие термодинамический анализ.
∆идкостное соединение можно исключить, сконструировав двойной элемент (два одинаковых элемента - навстречу).
Pt, H2 | HCl(m L)|AgCl, Ag...Ag, AgCl | HCl(m R) | H2, Pt
“акое исключение жидкостного соединени€ между полуэлементами в формуле элемента обозначаетс€ значком ||.

| –азность потенциалов гальванического элемента, измеренна€ при равновесии (ток не протекает) и между платиновыми клеммами, называетс€ электродвижущей силой (Ёƒ—). роме аббревиатуры используют обозначени€ э.д.с. или E. ћатематическа€ аддитивность потенциалов определ€ет результирующую разность потенциалов как сумму межфазных. |
| –ис. 5.8. ќпределение э.д.с. элемента по разност€м потенциалов. |
ѕроизвод€ суммирование, начина€ с правой платиновой клеммы, получаем:
 (5.15)
(5.15)
1) Ё.д.с. выражаетс€ в виде разности электродных потенциалов (5.16), которые кроме собственно межфазной разности потенциалов, включают контактную разность потенциала платиновой клеммы, если электрод не платиновый.
 (5.16)
(5.16)
ќтметим, что обозначени€ в скобках совмещают и названи€ веществ электродов, раствора и их агрегатное состо€ние.
2) ѕринимаетс€ соглашение (об€зательное дл€ последующего изложени€): знак э.д.с. всегда относитс€ к разности потенциалов E RЦ E L правого и
левого электродов в том виде, в которомзаписан гальванический элемент (при желательной, но не об€зательно аналогичной схеме его монтажа на подставке).
| ќбща€ запись Pt | ML | S | MR | Pt | : | ѕример
Pt | H2( ) | HCl(m) |AgCl, Ag | Pt ) | HCl(m) |AgCl, Ag | Pt
| ||
| L | R | L | R | |
ѕримечани€. Ќапомним, что межфазна€ граница в обозначении гальванического элемента, как правило, отмечаетс€ вертикальной чертой. »ногда внутреннюю межфазную границу, как в записи хлорсеребр€ного электрода, отмечают зап€той. «ап€той может быть отмечена и контактна€ платинова€ клемма. ƒополнительно в скобках конкретизируетс€:  , в единицах атм., давление дл€ газового электрода, а дл€ раствора Ц концентраци€, обычно в мол€льност€х m.
, в единицах атм., давление дл€ газового электрода, а дл€ раствора Ц концентраци€, обычно в мол€льност€х m.
|
|
|
ћаксимально положительна€ Ёƒ— будет наблюдатьс€ в том случае, если потребл€юща€ электроны восстановительна€ реакци€ 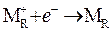 , создающа€ их дефицит и положительный потенциал на правом электроде, сочетаетс€ с окислительным высвобождением электронов
, создающа€ их дефицит и положительный потенциал на правом электроде, сочетаетс€ с окислительным высвобождением электронов  , дающим отрицательный потенциал на левом электроде.
, дающим отрицательный потенциал на левом электроде.
—ложение протекающих слева направо полуреакций естественно даст реакцию того же направлени€ и соответственно положительную Ёƒ—.
E > 0,  . (5.17+)
. (5.17+)
ќбратное протекание указанных процессов даЄт отрицательную Ёƒ—
E < 0,  . (5.17¯)
. (5.17¯)
ѕравило определени€ направлени€ спонтанной ќ¬– L + R+ = L+ + R по э.д.с.соответствующегогальваническогоэлемента L | L+ | R+ | R при использовании потенциалов восстановительных полуреакций. (¬еществам правого и левого электродов соответствуют символы R и L.):
1. «аписываем полуреакции на электродах в восстановительной форме с табличными значени€ми потенциалов (две первые строки). ѕри этом к правому электроду относитс€ та из них, котора€ по направлению совпадает с направлением анализируемой ќ¬– (втора€ строка). ќтносима€ к левому электроду сопр€женна€ окислительна€ полуреакци€, исход€ из записанной восстановительной еЄ формы, читаетс€ справа налево.
2. ƒл€ получени€ анализируемой реакции (треть€ и четверта€ строки слева) производим вычитание реагентов и продуктов полуреакции левого электрода из полуреакции правого, а также соответствующее вычитание потенциалов дл€ получени€ э.д.с. (треть€ строка справа).
3. –еакци€ протекает в направлении, указываемом знаком э.д.с. (четверта€ строка справа).
| Ц | L+ + eЦ = L | EL |
| R+ + eЦ = R | ER | |
| R+ Ц L+ = R Ц L | E = ER - EL | |
| R+ + L = R + L+ | E > 0 Ц вправо; E < 0 Ц влево. |
Ѕолее нагл€дный способ реализации правила (с использованием ранее введенного значка сопр€жени€ полуреакций) предусматривает при записи суммарной реакции сложение реагентов по Ђхвостамї стрелок и продуктов - по Ђостри€мї.
| + | L+ + eЦ R+ + eЦ | 
| L EL R ER |
| L + R+ = L++ R E = ER - EL |
ѕример - реакци€ коррозии железа в кислотных услови€х
Fe + 2 H+Cl¯ (aq) + 1/2 O2 = Fe2+Cl¯2(aq) + H2O
L: 1/2 Fe2+ + eЦ ← 1/2 Fe -0,44 ¬
R: H+ + eЦ + 1/4 O2 → 1/2 H2O 1,229 ¬
 E = 1,229 Ц (-0,44) =1,669 ¬
E = 1,229 Ц (-0,44) =1,669 ¬
ѕоложительна€ и значительна€ по величине Ёƒ— подтверждает активную роль этой реакции в коррозии железа.
”равнение Ќернста
–ассмотрим элемент L | L+ || R+ | R, в котором идет реакци€ L + R+ = L+ + R. ≈го э.д.с., ранее выраженна€ через разность электродных потенциалов (5.16), при последующей подстановке в них межфазных потенциалов (5.7) приводит к уравнению Ќернста (5.18).

 , (5.18)
, (5.18)
 . (5.19)
. (5.19)
Ёто важное дл€ практических приложений уравнение определ€ет концентрационную зависимость э.д.с. электрохимического элемента относительно стандартной э.д.с. (5.19), соответствующей концентраци€м, точнее активност€м компонентов в электролите ai = 1.
—тандартные электродные потенциалы
ѕотенциал одного электрода не входит в термодинамические выражени€ дл€ параметров, которые могут быть измерены на практике.
ѕоскольку имеет смысл говорить только о разности потенциалов, то одному электроду можно придать нулевое значение, и все другие электроды относить к нему. ¬ качестве электрода с нулевым потенциалом выбирают стандартный водородный электрод (—¬Ё). ≈го стандартный потенциал достигаетс€, когда активность ионов водорода равна единице; давление газообразного водорода, точнее его летучесть равна 1 атм, а температура составл€ет 298,15 (25 ∞—).
|
|
|
—¬Ё: H+( =1) | H2(f =1 атм), Pt; E ∞(H+ | H2) º 0. (5.20)
=1) | H2(f =1 атм), Pt; E ∞(H+ | H2) º 0. (5.20)
—тандартный потенциал других электродов может быть получен путем формировани€ гальванического элемента, в котором —¬Ё находитс€ слева, а исследуемый - справа. »оны и газы исследуемого электрода привод€тс€ к единичной активности и тогда E ∞( элемента ) = E ∞R Ц E ∞(—¬Ё) = E ∞R
насто€щему времени существуют таблицы стандартных электродных потенциалов. ѕричем современные их формы содержат стандартные потенциалы восстановлени€, соответствующие восстановительной форме записи электродной полуреакции. », тем не менее, дл€ контрол€ следует запомнить, что в такой таблице медь имеет положительный потенциал.
Cu+ + eЦ = Cu, E ∞(Cu+ | Cu) = 0,521 ¬
ћетод определени€ направлени€ протекани€ реакций по электродным потенциалам восстановлени€ - мощный количественный инструмент анализа окислительно-восстановительных реакций, учитывающий при необходимости концентрационные соотношени€ компонентов в растворе.
ѕример: ћожно ли анодным растворением (окислением) Zn вытеснить (восстановить) из раствора ионы 1) Cu2+, 2) Mg2+?
ј: 1/2 Zn2+ + eЦ ↔ 1/2 Zn; E ∞ = ‑0,763 ¬
: 1) 1/2 Cu2+ + eЦ → 1/2 Cu; ∆E ∞ = 0,337 - (‑0,763) > 0 Ц да.
2) 1/2 Mg2+ + eЦ ← 1/2 Mg; ∆E ∞ = -2,37 - (‑0,763) < 0 Ц нет.
ћеталлы, расположенные в пор€дке возрастани€ их стандартных восстановительных потенциалов образуют так называемый электрохимический р€д напр€жений металлов: Li, Rb, K, Е Mg, Al, Mn, Zn, Е H, Е Cu, Е Pt, Au.
Ј аждый металл способен вытесн€ть (восстанавливать) из растворов солей те металлы, которые сто€т в р€ду напр€жений после него.
Ј¬се металлы левее водорода, т.е. имеющие отрицательный потенциал, способны вытеснить водород из растворов кислот.
ѕроцедура определени€ стандартных электродных потенциалов, рассмотренна€ на примере хлорсеребр€ного электрода, состоит в измерении э.д.с. элемента Pt, H2 (1 атм) | HCl(m) | AgCl | Ag, Pt. ≈го э.д.с., равна€ разности (5.15) правого и левого электродных потенциалов (5.16) с учетом межфазных разностей потенциалов (5.11) в приложении к хлорсеребр€ному электроду и ((5.9), вариант б)) к водородному при соглашении (5.20), принимает вид:
 (5.21)
(5.21)
Ќапомним, что по теории ƒеба€-’юккел€ среднеионный коэффициент активности дл€ разбавленных растворов 1:1 определен формулой  , где m - мол€льность.
, где m - мол€льность.
Ёкспериментально исследу€ зависимость E (m) в области разбавленного раствора, методом математической обработки наход€т ее предельное (m → 0) значение E ∞. ѕосле этого можно экспериментально определить зависимость g± от m в более широкой области концентраций.






