ј14. ’арактерные химические свойства углеводородов: алканов, циклоалканов, алкенов, диенов, алкинов, ароматических углеводородов (бензола и толуола).
’имические свойства алканов
| “ип реакции | ”равнение |
| I. –еакции окислени€. | |
| 1. √орение: а) полное | CH4 + 2O2 → CO2 + 2H2O + Q 2—4Ќ10 + 13ќ2→ 8—ќ2 +10Ќ2ќ+Q |
| б) неполное. | 2CH4 + 3O2 → 2CO + 4H2O |
| 2. аталитическое окисление (различные катализаторы и t окислени€) а) метан Ц до метанола, метанал€ или муравьиной кислоты б) гомологи метана окисл€ютс€ с разрывом —-— цепи и образованием | 2CH4 + O2 → 2CH3OЌ CH4 + O2 → ЌCЌO + H2O 2CH4 + 3O2 → 2ЌCOOЌ+ 2H2O 2C4H10 + 5O2 → 4—Ќ3COOЌ+ 2H2O |
| II. «амещение. | |
| 1.√алогенирование (на свету или при t). ѕри хлорировании или бромировании алкана с вторичными или третичными атомами углерода легче всего идет замещение водорода у третичного атома, труднее у вторичного и еще труднее у первичного. ѕоэтому, например, при бромировании пропана основным продуктом реакции €вл€етс€ 2-бромпропан. | CH4 + Cl2 → CH3Cl + HCl ѕри достаточном количестве хлора реакци€ продолжаетс€ дальше: CH3Cl + Cl2 → CH2Cl2 +HCl CH2Cl2 + Cl2 →CHCl3 + HCl CHCl3 + Cl2 → CCl4 + HCl |
| 2.Ќитрование (реакци€ оновалова) | —2Ќ6 + ЌNќ3→ —2Ќ5Nќ2+ H2O |
| III. “ермические превращени€ алканов | |
| 1. ƒегидрирование гомологов метана | √омологи метана превращаютс€ в алкены: —2Ќ6 → —2Ќ4 + Ќ2 |
| 2. »зомеризаци€ (при нагревании и в присутствии хлорида алюмини€) |  —Ќ3-—Ќ2-—Ќ2-—Ќ3→ —Ќ3-—Ќ-—Ќ3
—Ќ3 —Ќ3-—Ќ2-—Ќ2-—Ќ3→ —Ќ3-—Ќ-—Ќ3
—Ќ3
|
| 3. ѕиролиз (разложение на простые вещества): а) полное б) неполное | 1000о — —Ќ4 → — + 2Ќ2 1500о— 2CH4 → —2Ќ2 + 3Ќ2 |
| 4. –азрушение — - — цепи (крекинг) (400-500 о—) | јлкан → новый алкан + алкен —20Ќ42 → —10Ќ22 + —10Ќ20 |
| IV. онверси€ метана при 800о— и в присутствии никелевого катализатора | —Ќ4 + Ќ2ќ → —ќ + 3Ќ2 |
| V. јроматизаци€ алканов (— ≥ 6) при нагревании и действии катализатора | —6Ќ14→ —6Ќ6 + 4Ќ2 |
| VI. ѕри о.у. не обесцвечивают бромную воду и раствор перманганата кали€ | - |
| VII. –еакции присоединени€ не характерны | - |
’имические свойства циклоалканов
| “ип реакции | ”равнение химической реакции |
| I. ќбщие с алканами (дл€ больших циклов — ≥ 5) протекают с сохранением цикла | |
| 1) «амещение: | |
| а) галогенирование | 
|
| б) нитрование | 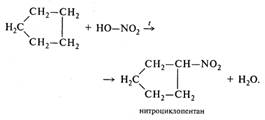
|
| 2) ƒегидрирование |  t,Pt
→ —6Ќ6 + 3Ќ2
циклогексан бензол
t,Pt
→ —6Ќ6 + 3Ќ2
циклогексан бензол
|
| 3) √орение | —6Ќ12+ 9O2 → 6CO2 + 6 H2O+Q |
| II. ќсобые свойства - отличные от алканов (дл€ малых циклов — = 3, 4), протекают с разрывом цикла | |
| 1) –еакции присоединени€ | |
| а) галогенирование | 
|
| б) гидрирование | |
| в) гидрогалогенирование |
’имические свойства алкенов
| “ип реакции | ”равнение химической реакции |
| I. ќкисление: 1) ѕолное (горение) | —2Ќ4 + 2ќ2 → —ќ2 + 2Ќ2ќ + Q |
| 2) Ќеполное (разрыв только π-св€зей) | ќкисление этилена перманганатом кали€ в нейтральной среде приводит к образованию двухатомного спирта Ц этиленгликол€. ”прощЄнно: CH2 = CH2 + ЌќЌ + [O] → CH2(OH) Ц CH2(OH) 3 CH2 = CH2 + 2KMnO4 + 4H2O → 3 CH2 Ц CH2 + 2MnO2 + 2KOH | | OH OH |
| 3) ∆Єсткое окисление | ѕри окислении алкенов кип€щим раствором KMnO4 в кислой среде происходит полное разрушение двойной св€зи и превращение атомов углерода, между которыми существует двойна€ св€зь, в атомы углерода карбоксильной группы с образованием карбоновых кислот или —ќ2: 5CH3-CH=CH-CH2-CH3 + 8KMnO4 + 12H2SO4 →5CH3COOH + 5C2H5COOH + 8MnSO4 + 4K2SO4 + 17H2O 5CH3CH=CH2 + 10KMnO4 + 15H2SO4 →5CH3COOH + 5CO2 + 10MnSO4 + 5K2SO4 + 20H2O ƒихромат кали€ в сернокислотной среде окисл€ет алкены аналогично. ≈сли атом углерода при двойной св€зи содержит 2 заместител€, то при его окислении образуетс€ кетон: 5(CH3)2-C=CH-CH3 + 6KMnO4 + 9H2SO4 →5CH3COOH + 5(CH3)2C=O + 6MnSO4 + 3K2SO4 + 9H2O ¬ щелочной среде: 3CH3-CH=CH-CH2-CH3 + 6KMnO4 + 10KOH →CH3COOK + C2H5COOK + 6H2O + 6K2MnO4 4CH3CH=CH2 + 10KMnO4 + 13KOH →CH3COOK + K2CO3 + 8H2O+ 10K2MnO4 |
| II. ѕрисоединение 1) √идрирование при о.у. | CH2 = CH2 + Ќ2 → CH3 Ц CH3 |
| 2) √идратаци€ | H2SO4
—Ќ2 = —Ќ2 + ЌќЌ → —Ќ3 Ц —Ќ2ќЌ
ѕрисоединение воды к несимметричному алкену происходит по правилу ћарковникова: Ђјтом водорода присоедин€етс€ к наиболее гидрированному атому углерода, а гидроксогруппа к менее гидрированному атому углеродаї.
 CH3-CH=CH2 + ЌќЌ → —Ќ3 Ц —ЌЦ —Ќ3
ќЌ CH3-CH=CH2 + ЌќЌ → —Ќ3 Ц —ЌЦ —Ќ3
ќЌ
|
| 3) √алогенирование | CH2 = CH2 + Br2 → CH2Br Ц CH2Br |
| 4) √идрогалогенирование | CH2 = CH2 + HBr → CH3Ц CH2Br ѕрисоединение галогеноводорода к несимметричному алкену происходит по правилу ћарковникова: Ђјтом водорода присоедин€етс€ к наиболее гидрированному атому углерода, а галогены к менее гидрированному атому углеродаї. —Ќ3 Ц CH = CH2 + HBr → CH3 Ц CH Ц CH3 | Br |
| III. ѕолимеризаци€ - процесс образовани€ полимера из низкомолекул€рных веществ, без выделени€ побочных продуктов | n CH2 = CH2 → (Ц CH2 Ц CH2 Ц)n - полиэтилен n CH2 = CЌ → (Ц CH2 Ц CH Ц)n - полипропилен | | CH3 CH3 |
| IV.–еакции замещени€: галогенирование | Ќаиболее легко замещаетс€ галогенами атом Ќ у первого атома —, счита€ от двойной св€зи: CH3- CH = CH2 + Cl2 → Cl - CH2- CH = CH2 + HCl |
|
|
|






