—ќƒ≈–∆јЌ»≈
1. ¬ведение.
2. «адание.
3. –асчет кривой комплексонометрического титровани€ раствора MgCl2 раствором трилона Ѕ, при PH=10
4. –асчет кривой комплексонометрического титровани€ раствора MgCl2 раствором трилона Ѕ, при PH= 8
¬ведение
омплексометрическим титрованием или комплексометрией называют титриметрические методы, основанные на реакци€х образовани€ растворимых комплексов ионов металлов с некоторыми ионами или молекулами. —оединение, которое образует комплекс с ионом металла, называетс€ лигандом. »он металла, взаимодейству€ с лигандом, образует ковалентные св€зи, электроны дл€ которых предоставл€ютс€ лигандами- донорами; ион металла-акцептор.
типичным лигандам относ€тс€ галогенид-ионы (F-,Cl-,Br-,I-),
азотосодержащие соединени€ (аммиак,пирид), гидроксил-ионы и др.
«адание: –ассчитать и построить кривые комплексонометрического титровани€ в 100 мл 0,05 ћ раствора MgCl2 и 100 мл 0,05 ћ раствора трилона Ѕ. ѕри PH=10 и PH=8. ѕри расчете кривой титровани€ необходимо рассчитать точки 90; 99; 99,9; точку эквивалентности и после точки эквивалентности (100,1;101; 110).
–асчет кривой титровани€ раствора MgCl2 раствором трилона Ѕ
1. –асчет условной константы. ¬еличину условной константы устойчивости комплекса Ёƒ“ј в буферном растворе с PH=10 можно получить из константы устойчивости и величины  дл€ Ёƒ“ј при PH =10
дл€ Ёƒ“ј при PH =10
 MgY2- =
MgY2- = 

 MgY2-= 3,5
MgY2-= 3,5  10-1
10-1  4,9
4,9  108 = 17,15
108 = 17,15  107
107
2. ¬ычисление pMg до точки эквивалентности. ƒо достижени€ точки эквивалентности обща€ концентраци€ Mg2+ равна сумме концентраций не оттитрованного избытка ионов Mg2+ и ионов Mg2+, образующихс€ за счет диссоциации комплекса. онцентраци€ комплекса равна общей концентрации Ёƒ“ј, добавленной к титруемому раствору металла. онцентраци€ металла, получаема€ за счет диссоциации комплекса мала по сравнению с концентрацией не закомплексованного иона Mg2+ и ею можно пренебречь.
[ Mg2+] = 
где — Ц концентраци€ Mg2+, получаема€ за счет диссоциации комплекса;
Vx, Nx , Vt, Nt Ц объемы и нормальности титруемого вещества и титранта.
50%: [ Mg2+] =  = 1,667
= 1,667  10-2 г-экв/л
10-2 г-экв/л
P[Mg2+] = 2-lg1,667=2-0,22=1,78
90%: [ Mg2+] =  = 2,63
= 2,63  10-3 г-экв/л
10-3 г-экв/л
P [Mg2+] =3Цlg2,63 = 3-0,42 = 2,58
99%: [ Mg2+] = 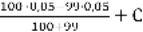 = 2,5
= 2,5  10-4 г-экв/л
10-4 г-экв/л
P [Mg2+] = 4Цlg2,5 = 4Ц0,4 = 3,6
99,9%: [ Mg2+] =  = 3
= 3  10-5 г-экв/л
10-5 г-экв/л
P [Mg2+] = 5Цlg3 = 5Ц0,48 = 4,52
3. «начение PMg в точке эквивалентности. ¬ этот момент концентраци€ MgY2- в растворе равна [MgY2-] =  =
=  =
=  = 0,025 г-экв/л
= 0,025 г-экв/л
 MgY2- =
MgY2- =  = 17,15
= 17,15  107
107
 17,15
17,15  107
107
 =
=  1,457
1,457  10-10
10-10
[Mg2+] =  = 1,207
= 1,207  10-5 г-экв/л
10-5 г-экв/л
PMg = 5 Ц lg1,207 = 5Ц0,08 = 4,92
PMg = 4,92
4. ¬ычисление PMg после точки эквивалентности. ѕосле точки эквивалентности концентраци€ ионов Mg2+ понижаетс€ за счет вли€ни€ на степень диссоциации комплекса избытка Ёƒ“ј. ¬еличину PMg рассчитывают из выражени€ дл€ условной константы устойчивости комплекса магний Ц Ёƒ“ј, подставл€€ в него отношение избытка Ёƒ“ј к концентрации комплекса.
|
|
|
100,1%:[Mg2+] =  = 5,83
= 5,83  10-6
10-6
PMg = 6 Ц lg5,83 = 6Ц0,76= 5,24
101%:[Mg2+] =  = 5,83
= 5,83  10-7
10-7
PMg = 7 Ц lg5,83 = 7Ц0,76 = 6,24
110%: [Mg2+] =  = 5,83
= 5,83  10-8
10-8
PMg = 8 Ц lg5,83 = 8Ц0,76 = 7,24
130%:[Mg2+] =  = 1,94
= 1,94  10-8
10-8
PMg= 8-lg1,94=8-0,29= 7,71
–асчет кривой титровани€ раствора MgCl2 раствором трилона Ѕ
1. –асчет условной константы. ¬еличину условной константы устойчивости комплекса Ёƒ“ј в буферном растворе с PH=10 можно получить из константы устойчивости и величины  4 дл€ Ёƒ“ј при PH =8.
4 дл€ Ёƒ“ј при PH =8.
 MgY2- =
MgY2- =  4
4 
 MgY2- = 5,4
MgY2- = 5,4  10-3
10-3  4,9
4,9  108 =2,646
108 =2,646  106
106
2. «начение PMg в точке эквивалентности. ¬ этот момент концентраци€ MgY2-в растворе равна [MgY2-] =  =
=  =
=  = 0,025 г-экв/л
= 0,025 г-экв/л
 2,646
2,646  106
106
 =
=  = 0,9448
= 0,9448  10-8
10-8
[Mg2+] =  = 0,972
= 0,972  10-4
10-4
PMg = 4Цlg0,972 = 4+0,012 = 4,012
PMg = 4,012
3. ¬ычисление PMg после точки эквивалентности. ѕосле точки эквивалентности концентраци€ ионов Mg2+ понижаетс€ за счет вли€ни€ на степень диссоциации комплекса избытка Ёƒ“ј. ¬еличину PMg рассчитывают из выражени€ дл€ условной константы устойчивости комплекса магний Ц Ёƒ“ј, подставл€€ в него отношение избытка Ёƒ“ј к концентрации комплекса.
100,1%:[Mg2+] =  = 377,9289
= 377,9289  10-6
10-6
PMg = 6-lg377,9289 = 6-2,57 = 3,43
101%:[Mg2+] =  = 37,7929
= 37,7929  10-6
10-6
PMg = 6-lg37,7929 = 6-1,57 = 4,43
110%:[Mg2+] =  = 3,7793
= 3,7793  10-6
10-6
PMg = 6-lg3,7793 = 6-0,57 = 5,43
130%: [Mg2+] =  = 1,26
= 1,26  10-6
10-6
PMg = 6-lg1,26 = 6-0,1 = 5,9
ѕри расчете концентраци€ металла с точностью 0,1% оказалась выше, чем в точке эквивалентности и после, поэтому будем считать с погрешностью 1%.






