”слови€ проведени€ опыта.
1. ƒл€ проведени€ реакции беретс€ свежеприготовленный раствор хлорида олова (II).
2. Ќе следует брать большого избытка щелочи.
3. ѕроведению реакции мешают катионы Ag+, Hg2+ и др.
„астные реакции катиона Mg2+
1. √идроксиды кали€ и натри€ образуют с катионом Mg2+ белый аморфный осадок гидроксида магни€ Mg(OH)2, растворимый в кислотах и сол€х аммони€.
ќпыт. ¬ первую пробирку возьмите 4 капли раствора соли магни€, прибавьте 4 капли насыщенного раствора хлорида аммони€. ¬о вторую пробирку возьмите 4 капли раствора соли магни€ и прибавьте 4 капли воды (чтобы концентраци€ растворов была одинакова). «атем в обе пробирки прибавьте осадитель Ц гидроксид аммони€. ѕочему не выпадает осадок в первой пробирке? ƒайте объ€снение.
2. √идрофосфат натри€ Na2HPO4 дает с катионом магни€ в присутствии гидроксида и хлорида аммони€ белый кристаллический осадок фосфата магни€-аммони€ MgNH4PO4:
MgSO4 + Na2HPO4 + NH4OH Ѓ MgNH4PO4¯ +Na2SO4 + H2O
Mg2+ + HPO42- + NH4OH Ѓ MgNH4PO4 + H2O
’лорид аммони€ добавл€ют, чтобы не выпал аморфный осадок гидроксида магни€ Mg(OH)2.
ќпыт. ¬озьмите 3-4 капли раствора соли магни€ и смешайте с 4-5 капл€ми 2Ќ раствора сол€ной кислоты и 3-5 капл€ми раствора гидрофосфата натри€ Na2HPќ4. ѕосле этого прибавьте к раствору по одной капле 2 Ќ раствора аммиака, перемешива€ раствор после каждой капли. ¬начале аммиак нейтрализует прибавленную кислоту, причем образуетс€ хлорид аммони€, преп€тствующий образованию гидроксида магни€. ѕосле окончани€ реакции выпадает характерный кристаллический осадок Ц фосфат магни€-аммони€ MgNH4PO4.
”слови€ проведени€ опыта.
1. –еакци€ проводитс€ в аммиачной среде при рЌ=8.
2. »збыток катионов NH4+ мешает выпадению осадка MgNH4PO4.
3. Ќе следует брать избыток сол€ной кислоты.
4. атионы всех аналитических групп, кроме первой, могут мешать проведению реакции.
3. ћикрокристаллоскопическа€ реакци€.
ќпыт. аплю раствора соли магни€, содержащую немного хлорида аммони€ поместите на предметное стекло и обработайте в парах 25% раствора аммиака. «атем поместите в каплю кристаллик гидрофосфата натри€ Na2HPO4× 12Ќ2ќ и рассмотрите кристаллы под микроскопом.
4. ћагнезон 1(пара-нитробензолазорезорцин) или магнезон II (пара-нитробензолазо-a-нафтол)
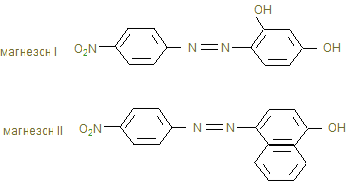
в щелочной среде дает красную или красно-фиолетовую окраску. Ёта реакци€ основана на свойстве гидроксида адсорбировать некоторые красители.
ќпыт. Ќа фарфоровую пластинку (предметное стекло или пробирку) поместите 1-2 капли анализируемого на катион Mg2+ раствора и добавьте 1-2 капли щелочного раствора реактива. ѕо€вл€етс€ син€€ окраска или синий осадок. ≈сли раствор имеет сильнокислую реакцию, то по€вл€етс€ желта€ окраска. ¬ данном случае надо добавить несколько капель щелочи.
”слови€ проведени€ опыта.
1. –еакцию необходимо проводить в щелочной среде при рЌ>10.
2. –еакции мешает наличие солей аммони€.
„астные реакции катиона Sb3+
√идролиз. ѕри действии воды на соли сурьмы они подвергаютс€ гидролизу в большей степени, чем соли висмута:
|
|
|
SbCl3 +H2O ЂSbOCl¯ +2HCl
ќпыт. Ќесколько капель раствора соли сурьмы разбавьте водой - выпадает белый осадок хлористого стибила или хлористого антимонила.
”слови€ проведени€ опыта.
1. рЌ среды должен быть не меньше 3-4. ¬ избытке кислоты осадок может не выделитьс€.
2.Ќагревание способствует выпадению осадка
3.Ќаличие винной кислоты в растворе преп€тствует выпадению осадка (в отличие от соединений висмута).
2. “иосульфат натри€ Na2S2O3 дает с катионом Sb3+ при нагревании красный осадок сероокиси сурьмы (III) Sb2O2S2:
2SbCl3 +2Na2S2O3 + 3H2O Ѓ Sb2OS2¯ +2H2SO4 + 4NaCl +2HCl
2Sb3+ +2S2O32- + 3H2O Ѓ Sb2OS2 +2SO42-+ 4NaCl +6H+
ѕри наличии ионов Bi3+ выпадает черный осадок, который будет маскировать окраску сероокиси сурьмы.
ќпыт. ¬ пробирку налейте 2-3 капли раствора хлорида сурьмы (III), добавьте каплю серной кислоты и 5-6 капель воды, бросьте кристалик тиосульфата натри€ и нагрейте. ¬ыпадает красный осадок сероокиси сурьмы.
”слови€ проведени€ опыта.
1. »збыток серной кислоты разлагает реактив с образованием оксида серы (IV) SO2 и серы.
2. Ќаличие катионов —u2+, Hg2+ и др., образующих труднорастворимые сульфиды, мешает проведению реакции.
3. ¬осстановление иона Sb3+до металлической сурьмы. ¬осстановить катионы Sb3+ до металлической сурьмы можно металлами, сто€щими в р€ду напр€жени€ левее сурьмы.
ќпыт. Ќа хорошо очищенную наждачной бумагой цинковую, алюминивую или железную пластинку нанесите каплю подкисленного сол€ной кислотой анализируемого раствора. ѕосле некоторого времени поверхность пластины под каплей становитс€ черной (выделение металлической сурьмы).
3Zn +2SbCl3 Ѓ 2Sb¯ + 3ZnCl2
”слови€ проведени€ опыта.
1. –аствор, содержащий ионы Sb3+, должен иметь реакцию при рЌ=1-2.
2. »спытуемый раствор не должен содержать сильных окислителей.
3. ѕочернение пластины происходит через 2-3 минуты.
„астные реакции катиона Sb5+
1. √идролиз. ѕри разбавлении растворов солей сурьмы водой образуетс€ белый осадок (основна€ соль сурьмы), растворимый в избытке сол€ной кислоты:
[SbCl6]- + 2H2O Ѓ SbO2Cl¯ + 5Cl- + 4H+
2. √идроксиды натри€, кали€ и аммиак дают с катионом Sb5+ белый осадок метасурьм€ной кислоты:
[SbCl6]- + 5ќЌ- Ѓ HSbO3¯ +6Cl- + 2H2O
3. ћеталлы Ц цинк, олово, магний, железо Ц действуют на катион Sb5+ так же, как и на катион Sb3+.
4. ћетиловый фиолетовый в сол€нокислых средах с катионом дает фиолетовое окрашивание.
ќпыт. ¬ пробирку возьмите 2-3 капли анализируемого раствора, 2-3 капли сол€ной кислоты и 1-2 капли раствора нитрита натри€ NaNO2. «атем добавьте 3 капли насыщенного раствора мочевины и слегка нагрейте. 2 капли приготовленного раствора поместите в пробирку и налейте 1 мл водного раствора реагента. ѕри наличии Sb5+ раствор приобретает фиолетово-синюю окраску.
”слови€ проведени€ опыта.
1. ƒл€ окислени€ ионa Sb(III) добавл€ют раствор нитрита натри€ (лучше брать кристаллический)
2. ƒл€ удалени€ избытка иона NO2 - добавл€етс€ насыщенный раствор мочевины.
3. Ќеобходимо проведение контрольного опыта.
ќпыт. ¬ пробирку налейте 2-3 капли дистиллированной воды, добавьте 2-3 капли хлороводородной кислоты (d=1,19), 1-2 капли раствора нитрита натри€ и 3 капли насыщенного раствора мочевины и слегка нагрейте.
ѕосле этого 2 капли приготовленного раствора поместите в пробирку и добавьте 2 мл водного раствора реагента. –аствор не должен иметь фиолетовой окраски.
|
|
|
онтрольна€ задача
“≈ћј: јнализ смеси катионов п€той группы
ѕри систематическом ходе анализа катионов п€той группы учитываетс€ некоторые особенности их соединений: гидролизуемость солей висмута и сурьмы, растворимость основных солей сурьмы в винной кислоте, нерастворимость оксида марганца в разбавленной азотной кислоте, растворимость гидроксида магни€ в растворе хлорида аммони€.
ќткрытие катионов Fe2+, Fe3+.
атионы Fe2+ обнаружают раствором гексациано-(III) феррата кали€, а катионы Fe3+ - раствором гексациано-(II) феррата кали€ в сол€нокислой среде в присутствии катионов п€той группы.
ќткрытие катионов Sb3+, Bi3+.
ѕри наличии осадка его раствор€ют в сол€ной кислоте, довод€т рЌ раствора до 4-6. «атем добавл€ют п€тикратный объем дистиллированной воды. ќбразовавшийс€ осадок основных солей сурьмы и висмута фильтруют, обрабатывают его раствором винной кислоты (1н). ќсновные соли и гидроксид сурьмы при этом раствор€ют в винной кислоте Ц производные висмута остаютс€ в осадке. ¬ полученном виннокислом растворе открывают сурьму (III) любой характерной реакцией, лучше сероводородной водой. ќсадок, содержащий основные соли висмута, промывают дистиллированной водой и раствор€ют в концентрированной сол€ной кислте. атионы висмута открывают хлоридом олова(II) в щелочном растворе или другими частными реакци€ми.
ќткрытие катионов Mg2+, Mn2+, Fe3+.
‘ильтрат, содержащий катионы Fe2+, Mg2+, Mn2+, Fe3+ обрабатывают раствором гидроксида натри€ или кали€, добавл€ют перекись водорода, нагревают и фильтруют. ѕолученный осадок гидроксида железа (III), оксида марганца и гидроксида магни€ обрабатывают раствором хлорида аммони€ дл€ растворени€ гидроксида магни€. ќставшийс€ осадок гидроксида железа (III), оксида марганца обрабатывают разбавленной азотной кислотой дл€ растворени€ гидроксида железа (III). ¬ полученном растворе открывают катионы Fe3+ характерными реакци€ми.
ќсадок оксида марганца раствор€ют в сол€ной кислоте или серной кислоте, и в полученном растворе открывают катионы Mn2+ персульфатом аммони€ (окиление Mn2+до MnO4-), ведут при нагревании, добавив каплю раствора нитрата серебра. атионы Mn2+ можно открывать дробным путем этой же реакцией.
атионы магни€ обнаруживают действием Na2HPO4 или магнезон 1 в фильтрате (III).
раствору солей железа(III) приливают раствор аммиакa до по€влени€ осадка, затем приливают несколько капель сол€ной кислоты и кип€т€т. атион Fe3+ переходит в раствор, где его обнаруживают характерными реакци€ми.
—хема систематического хода анализа катионов п€той группы.
| 1. ≈сли катионы п€той группы наход€тс€ в виде осадка гидроксидов, то его раствор€ют в сол€ной кислоте. | ||
| 2. ¬ отдельных пробах открывают ион железа (II) сK3[Fe(CN)6] и ион железа (III) с K4[Fe(CN)6] | ||
| 3. ’лороводородный раствор нейтрализуют 0,5 Ќ раствором NaOH до по€влени€ слабой мути, которую потом раствор€ют добавлением нескольких капель HCl. –азбавл€ют дес€тикратным раствором дистиллированной воды, фильтруют. | ||
| 4. ќсадок (I) SbOCl, BiOCl обрабатывают в пробирке винной кислотой и фильтруют. | 5. ‘ильтрат (I) Fe2+, Mg2+, Mn2+, Fe3+ - все катионы осаждают NaOH, добавл€ют Ќ2ќ2, нагревают и фильтруют. | |
| 8. ќсадок (III) Fe(OH)3, MnO2, Mg(OH)2 обрабатыают раствором хлорида аммони€ NH4Cl. | ||
| 9. ‘ильтрат (III) MgCl2 открывают с: а) Na2HPO4, б) магнезоном 1 | 10. ќсадок (IV) Fe(OH)3, MnO3 раствор€ют в разбавленной азотной кислоте. | |
| 6. ќсадок (II) BiOCl промывают водой, раствор€ют в HNO3 и открывают Bi3+ характерной реакцией. | ||
| 12. ќсадок (V) MnO2 раствор€ют в сол€ной кислоте и обнаруживают с (NH4)2S2O8. | 11. ‘ильтрат (IV) Fe3+, Fe2+ открывают KSCN. | |
| 7. ‘ильтрат (II) C4H4O6(SbO). »з данного раствора открывают сурьму (III) или сурьму (V) характерными реакци€ми. |
Ћабораторна€ работа є7
“≈ћј: Ўеста€ аналитическа€ группа катионов
(группы гидроксидов, раствор€емых в избытке раствора аммиака)
|
|
|
шестой аналитической группе относ€тс€ катионы Cu2+,Hg2+, Cd2+, Co2+, Ni2+.
’от€ элементы их расположены в разных группах ѕериодической системы ƒ.». ћенделеева: медь Ц в первой, кадмий и ртуть во второй, кобальт и никель в восьмой; все эти катионы характеризуют способность к комплексообразованию. »х гидроксиды раствор€ютс€ в избытке аммиака с образованием аминокомплексов различного состава.
√рупповым реагентом на катионы шестой группы €вл€етс€ гидроксид аммони€ в избытке. –астворы солей меди, кобальта и никел€ окрашены, кадми€ и ртути (II) бесцветны.
√идроксиды кали€ и натри€ с растворами, содержащими катионы Cd2+, Cu2+, Ni2+ дают аморфные осадки гидроксидов, с катионами Hg2+ - оксиды, с катионами —о2+ - основные соли:
CuSO4 + 2KOH Ѓ Cu(OH)2¯ + K2SO4
Cu2+ + 2OH- Ѓ Cu(OH)2
CdCl2 + 2KOH Ѓ Cu(OH)2¯ + KCl
Cd2+ + 2OH- Ѓ Cd(OH)2
NiCl2 + 2KOH Ѓ Ni(OH)2¯ + KCl
Ni2+ + 2OH- Ѓ Ni(OH)2
HgCl2 + 2KOH Ѓ HgO¯ + 2KCl +H2O
Hg2+ + 2OH- Ѓ HgO + H2O
CoCl2 + KOH Ѓ CoOHCl¯ + KCl
Co2+ + OH- Ѓ CoOHCl
¬се эти осадки растворимы в кислотах (сол€ной, азотной и серной) и в избытке аммиака, кроме соединений ртути (II), которые раствор€ютс€ в 25% растворе аммиака лишь при добавлении солей аммони€.
ќпыт. ¬ 5 пробирок налейте по 3 капли раствора соответствующей соли, добавьте по 3 капли раствора щелочи и перемешайте стекл€нной палочкой. ќбратите внимание на характер и цвет осадка. «атем добавьте в каждую пробирке по 8 капель хлороводородной кислоты, перемешайте стекл€нной палочкой, обратите внимание на цвет и растворимость осадка.
–аствор аммиака (не в избытке) взаимодействует с катионом шестой группы с образованием разных соединений:
2CuSO4 + 2NH4OH Ѓ (CuOH)2SO4¯ + (NH4)2SO4
2Cu2+ + SO42-+ 2NH4OH Ѓ (CuOH)2SO4 +2NH4+
CdCl2 + 2NH4OH Ѓ Cd(OH)2¯ + 2NH4Cl
Cd2+ + 2OH- Ѓ Cd(OH)2
HgCl2 + 2NH4OH Ѓ [NH2Hg]Cl¯ + 2NH4Cl +2H2O
HgCl2 + 2NH4OH Ѓ [NH2Hg]Cl¯ + NH4+ + Cl- +2H2O
NiCl2 + NH4OH Ѓ NiOHCl¯ + NH4Cl
CoCl2 + NH4OH Ѓ CoOHCl¯ + NH4Cl
онцентрированный раствор аммиака в избытке дает растворимые соли:
—uSO4 + 4NH4OH Ѓ [Cu(NH3)4]SO4 +4H2O
—u2+ + 4NH4OH Ѓ [Cu(NH3)4]2+ +4H2O
HgCl2 + 4NH4OH Ѓ [Hg(NH3)4]Cl2 +4 H2O
Hg2+ + 4NH4OH Ѓ [Hg(NH3)4]2+ +4 H2O
CdCl2 + 4NH4OH Ѓ [Cd(NH3)4]Cl2 +4 H2O
Cd2+ + 4NH4OH Ѓ [Cd(NH3)4]2+ +4 H2O
NiCl2 + 6NH4OH Ѓ [Ni(NH3)6]Cl2 +6 H2O
Ni2+ + 6NH4OH Ѓ [Ni(NH3)6]2+ +6 H2O
CoCl2 + 6NH4OH Ѓ [Co(NH3)6]Cl2 +6 H2O
Co2+ + 6NH4OH Ѓ [Co(NH3)6]2+ +6 H2O
ќбразование аммиаката ртути (II) и кобальта происходит при нагревании и добавлении к смеси солей хлорида аммони€ NH4—l (избыток катиона NH4+ сдвигает реакцию вправо).
–астворы аммиакатов довольно устойчивы, за исключением аммиаката кобальта, который постепенно под вли€нием кислорода из воздуха переходит в аммиакат кобальта (III), имеющего вишнево-красный цвет. ¬ присутствии окислителей реакци€ протекает мгновенно.
ќпыт. ¬озьмите 5 пробирок, внесите в каждую из них по 3 капли раствора солей меди (II), ртути(II), кадми€, никел€, кобальта. ƒобавьте сначала по 3 капли 25% раствора аммиака, перемешайте стекл€нной палочкой. ќбратите внимание на цвет осадка, затем в каждую пробирку внесите по 6 капель концентрированного раствора аммиака, снова перемешайте стекл€нной палочкой, кроме того, добавьте в растворы, содержащие катионы Hg2+, Co2+ , несколько капель кристаллов хлорида аммони€ NH4Cl.
—равните цвет осадков с окраской растворов комплексных солей.
—ероводород из нейтральных растворов осаждает все катионы шестой аналитической группы в виде сульфидов. —ульфиды меди(II), ртути(II), никел€ и кобальта черного цвета, сульфиды кадми€ Ц желтого цвета.
—ульфид кобальта и сульфид никел€ в кислых растворах в осадок не выпадает. —ульфид кадми€ выпадает в осадок только в слабокислой, лучше уксуснокислой среде, а сульфид меди (II) не раствор€етс€ при нагревании в разбавленной азотной кислоте. —ульфид ртути (II) не раствор€етс€ в разбавленных кислотах, но раствор€етс€ при нагревании в концентрированной азотной кислоте и царской водке.
|
|
|
—ероводород €довит! –аботать в выт€жном шкафу!
ќпыт. ¬озьмите 5 пробирок и в каждую налейте по 3-4 капли раствора солей меди (II), ртути (II), кадми€, никел€ и кобальта, и добавьте по 5-8 капель сероводородной воды. ƒл€ растворени€ осадков возьмите по 5-7 капель кислоты и внесите их в пробирки, тщательно перемешайте стекл€нной палочкой.
„астные реакции катиона Cu2+
1. ¬одный раствор аммиака, вз€тый в некотором избытке, дает аммиакат меди, имеющий сине-фиолетовую окраску:
CuSO4 + 4NH4OH Ѓ [Cu(NH3)4]SO4 +4H2O
ќпыт. ¬ фарфоровую чашку поместите 4 - 5 капель раствора соли меди, осторожно выпарьте досуха на асбестированной сетке, охладите и на периферическую часть п€тна нанесите каплю концентрированного раствора аммиака. ѕо€вление интенсивной синеЦфиолетовой окраски говорит о присутствии катиона Cu2+. Ёту реакцию удобно использовать дл€ открыти€ катиона Cu2+ в присутствии катионов всех групп.
2. “иосульфат натри€ Na2S2O3, прибавленный к подкисленному раствору соли меди, обесцвечивает раствор, так как образуетс€ комплексна€ соль. ѕри нагревании полученного раствора образуетс€ темно-бурый осадок сульфида меди Cu2S.
–еакции катиона Cu2+ может протекать с тиосульфатом натри€ с образованием различных продуктов в зависимости от количества реагента. ѕи избытке:
2CuSO4 + 2Na2S2O3 Ѓ Na2SO4 + Na2S4O6 + Cu2SO4
Cu2SO4+ Na2S2O3 Ѓ Na2SO4 + Cu2S2O3
Cu2S2O3 + Na2S2O3 Ѓ Na2[Cu2(S2O3)2]
Na2[Cu2(S2O3)2] + H2SO4 Ѓ Na2SO4 + H2[Cu2(S2O3)2]
H2[Cu2(S2O3)2] Ѓ H2SO4 + SO2≠ + Cu2S¯ + S¯
__________________________________________________________
2CuSO4 + 4Na2S2O3 Ѓ 3Na2SO4 + Na2S4O6 + S¯ + Cu2S¯ + SO2≠
ѕри эквивалентных соотношени€х и недостатке реагента:
CuSO4 + Na2S2O3 Ѓ CuS2O3 + Na2SO4
CuS2O3 + H2O Ѓ CuS¯ + H2SO4
ќпыт. Ќалейте в пробирку 2-3 капли раствора сульфата меди, добавьте 4-5 капель воды, 2-3 капли раствора серной кислоты и добавьте 2-3 кристаллика тиосульфата натри€. ѕеремешайте стекл€нной палочкой и нагрейте. ќбразуетс€ темно-бурый осадок сульфида меди (I) и серы.
јзотна€ кислота должна отсутствовать, она окисл€ет тиосульфат натри€ до Na2SO4 и S.
ћеталлический алюминий, железо и цинк восстанавливают катион Cu2+ до свободного металла, имеющего вид красной губчатой массы.
Cu2+ + Zn Ѓ Cu + Zn2+
ќпыт. Ќа обезжиренную и зачищенную металлическую пластину (алюминиевую, железную или цинковую) нанесите каплю раствора соли меди, подкисленную серной кислотой. „ерез некоторое врем€ по€вл€етс€ красноватое п€тно меди.
”слови€ проведени€ опыта.
1. –еакцию следует проводить в кислой среде при рЌ=1-2.
2. ¬ присутствии азотной кислоты осаждение меди не происходит.
3. –еакци€ с гексацианоферратом(II) кали€.
атионы Cu2+ образуют с ферроцианидом кали€ K4[Fe(CN)6] в слабокислой средекрасно-коричневый осадок гексацианоферрата(II) меди Cu2[Fe(CN)6]
2Cu2+ + [Fe(CN)6]4- Ѓ Cu2[Fe(CN)6]
ќсадок не раствор€етс€ в в разбавленных кислотах, но раствор€етс€ в 25%-м водном аммиаке:
Cu2[Fe(CN)6] + 12NH3 + 4H2O Ѓ(NH4)4[Fe(CN)6] + [Cu (N3)4](OH)2
ћешают катионы, также образующие окрашенные осадки феррацианидов (Fe3+, Co2+, Ni2+).
–еакци€ катионов меди(II) с ферроцианидом кали€ можно проводить капельным методом на фильтровальной бумаге.
„астные реакции катиона Hg2+
—оли ртути €довиты!
1. —ероводород и тиосульфат натри€ Na2S2O3 в кислой среде с сол€ми ртути (II) при нагревании дают черный осадок сульфида ртути (II) HgS, нерастворимый в разбавленной азотной кислоте:
HgCl2 + H2S Ѓ HgS¯ + 2HCl
2. »одид кали€ образует с катионом Hg2+ красный осадок иодида ртути HgI2, растворимый в избытке реагента.
Hg(NO3)2 + 2KI Ѓ HgI2¯ + KNO3; Hg2+ + 2I- Ѓ HgI2
HgI2 + 2KI Ѓ K2[HgI4]; HgI2 + 2I- + 2K+ Ѓ K2[HgI4]
ќпыт. а) в пробирку налейте 4-5 капель раствора нитрата ртути (II) Hg(NO3)2 и осторожно опустите палочку, моченную раствором иодида кали€ KI. ¬округ палочки образуетс€ €рко-красное п€тно иодида ртути, которое быстро исчезает.
б)  Ќа полоску фильтровальной бумаги нанесите одну каплю разбавленного раствора иодида кали€. «атем возьмите пипеткой раствор соли ртути (II), коснитесь осторожно пипеткой центра п€тна и подержите 5-6 секунд. ќбразуетс€ красное п€тно.
Ќа полоску фильтровальной бумаги нанесите одну каплю разбавленного раствора иодида кали€. «атем возьмите пипеткой раствор соли ртути (II), коснитесь осторожно пипеткой центра п€тна и подержите 5-6 секунд. ќбразуетс€ красное п€тно.
Ётими реакци€ми можно определить катион Hg2+ в присутствии катионов всех аналитических групп, кроме Ag+, Pb2+, которые предварительно удал€ют из анализируемого раствора, добавл€€ смесь растворов хлорида кали€ и сульфата кали€.
|
|
|
”слови€ проведени€ опыта.
1. –еакци€ протекает при рЌ< 6.
2. »збыток иодида кали€ приводит к растворению осадка.
3. атионы Ag+, Pb2+ и др., образующие с KI осадки, мешают проведению реакции, поэтому их предварительно необходимо удалить.
3. ’лорид олова SnCl2 восстанавливает Hg2+ до [Hg2]2+, а затем до металлической ртути:
2HgCl2 + SnCl2 Ѓ Hg2Cl2¯ + SnCl4
Hg2Cl2 + SnCl2 Ѓ 2Hg¯ + SnCl4
ќпыт. Ќа полоску фильтровальной бумаги нанесите каплю свежеприготовленного сол€нокислого раствора хлорида олова SnCl2 и в центр п€тна поместите сначала каплю раствора нитрата серебра, а затем каплю раствора соли ртути(II).
¬ присутствии катионов Hg2+ образуетс€ черное п€тно:
2Hg2+ + Sn2+ + Ag+ + 2Cl- Ѓ Sn+4 + Ag¯ + Hg2Cl2¯
”слови€ проведени€ опыта.
1. –еакцию провод€т при рЌ< 5.
2. ѕри выполнении опыта необходимо использовать свежеприготовленный раствор хлорида олова.
3. —ильные окислители мешают протеканию реакции.
4. ¬осстановление катиона Hg2+ до свободной ртути можно проводить металлической медью.
ќпыт. Ќа медную пластинку нанесите каплю испытуемого раствора. „ерез 4-5 минут на поверхности пластинки по€вл€етс€ черное п€тно металлической ртути.
”слови€ проведени€ опыта.
1. ¬ыполнению этой реакции мешают ионы Ag+, Bi3+, [Hg2] 2+, Sb+3,
2. ѕри наличии в испытуемом растворе Sb+3 , Bi3+ реакци€ протекает в присутствии анилина.
„астные реакции катиона Cd2+
1. —ероводород в уксуснокислой среде с катионом Cd2+дает желтый осадок сульфида кадми€:
H2S + CdCl2 Ѓ 2HCl + CdS¯
S 2-+ Cd2+ Ѓ CdS¯
“ак как открытию катиона Cd2+ мешают другие катионы шестой группы, то реакцию провод€т следующим образом.
ќпыт. ¬ пробирку возьмите 3-4 капли раствора, содержащего катион Cd2+, добавьте 6 капель серной кислоты и 2-3 кристаллика тиосульфата натри€ и нагрейте на вод€ной бане 2-3 минуты. ѕри этом сульфид ртути HgS и сульфид меди Cu2S выпадают в осадок. ¬ растворе остаютс€ катионы Co2+, Ni2+, Cd2+. ќсадок отделите. ¬ другую пробирку налейте 5-6 капель сероводородной воды и добавьте 2-3 капли фильтрата.
ѕри наличии катиона Cd2+ сейчас же выпадает желтый осадок сульфида кадми€ CdS. ѕроверьте его растворимость в сол€ной и уксусной кислотах.
”слови€ проведени€ опыта.
1. –еакцию образовани€ сульфида кадми€ провод€т в уксуснокислой среде.
2. ќкислители мешают осуществлению реакции.
2. “иомочевина образует с сол€ми кадми€ легкорастворимые комплексные соли:
CdCl2 + 4 CS(NH2)2 Ѓ [Cd(CSN2H4)4]Cl2
омплексные соли кадми€ легко разлагаютс€ сероводородом даже при избытке тиомочевины. омплексы других катионов устойчивы.
ќпыт. ¬ пробирку налейте 3-4 капли соли кадми€, добавьте 3-4 кристаллика тиомочевины, перемешайте палочкой, дайте посто€ть 3 минуты и затем добавьте 5-6 капель свежеприготовленной сероводородной воды.
¬ присутствии катиона Cd2+ выпадает желто-оранжевый осадок. Ёта реакци€ позвол€ет открыть Cd2+ в присутствии ионов Cu2+, которые дают прочный тиомочевинный комплекс.
3. ќткрытие Cd2+ при помощи KI. атионы кадми€ с избытком KI и NH4ќЌ образует белый осадок комплексной соли [Cd(NH3)4]I2. ƒл€ открыти€ катионов кадми€ к раствору соли кадми€ приливают раствор аммиака до полного растворени€ образовавшегос€ вначале осадка, затем Ц небольшое количество 30-40% свежеприготовленного раствора KI. ѕри этом через некоторое врем€ из раствора выдел€етс€ белый осадок йодистого тетраммина кадми€:






