1. –ассчитайте изменение энтропии, энтальпии и энергии √иббса при с.у. дл€ реакции гидролиза мочевины —ќ(NЌ2)2(ж) + Ќ2ќ(ж) = —ќ2(г) + 2 NЌ3(ж).
2. –ассчитайте изменение энтропии, энтальпии и энергии √иббса реакции образовани€ глицилглицина 2 —Ќ2(NЌ2)—ќќЌ(ж) → —Ќ2(NЌ2)—ќNЌ—Ќ2—ќќЌ(ж) + Ќ2ќ(ж) при 298 .
3. ƒл€ реакции 2 NO2(г) ↔ N2ќ4(г) вычислите ΔG∞р и температуру, при которой оба направлени€ процесса равноверо€тны.
4. –ассчитайте изменение энтропии, энтальпии и энергии √иббса реакции окислени€ этанола в уксусный альдегид —2Ќ5ќЌ(ж) + ќ2(г) ↔ —Ќ3—Ќќ(г) + Ќ2ќ(ж) при с.у.
5. √лицерин Ц один из продуктов метаболизма, который окончательно превращаетс€ в организме в —ќ2(г) и Ќ2ќ(ж). –ассчитайте изменение энтропии, энтальпии и энергии √иббса дл€ этой реакции при 298 .
6. –еакцией—6Ќ12ќ6(ж) → —3Ќ7—ќќЌ(ж) + 2 —ќ2(г) + 2Ќ2(г) выражаетс€ один из путей метаболизма глюкозы. –ассчитайте изменение энтропии, энтальпии и энергии √иббса дл€ этой реакции при с.у.
7. –ассчитайте изменение энтропии, энтальпии и энергии √иббса при с.у. дл€ реакции† —ќ(г) + —l2(г) ↔ —ќ—l2(г).
8. –ассчитайте изменение энтропии, энтальпии и энергии √иббса дл€ реакции фотосинтеза, протекающей в организме:†† 6 —ќ2(г) + 6 Ќ2ќ(ж) ↔ —6Ќ12ќ6(ж) + 6ќ2(г) при 298 .
9. ¬ычислите среднюю скорость реакции ј → ¬, если начальна€ концентраци€ исходного вещества 6 моль/л, а через 2 мин Ц 2 моль/л.
10. ¬о сколько раз увеличитс€ скорость реакции, протекающей при 298 , если энерги€ активации (Ea) уменьшить на 4 кƒж/моль?
11. ¬ычислите энергию активации в указанном интервале температур, если константа скорости разложени€ оксида азота (V) при 35 о— равна 8,76Ј10-3 мин-1, а при 45о— увеличиваетс€ до 2,99Ј10-2 мин-1.
12. ѕри лечении онкологических заболеваний в опухоль ввод€т препарат, содержащий радионуклид иридий -192. –ассчитайте, кака€ часть введенного радионуклида останетс€ в опухоли через 10 суток, если его период полупревращени€ составл€ет 74,08 суток.
13. ¬ычислите температурный коэффициент константы скорости разложени€ пероксида водорода в температурном интервале 25-55о— при Ea = 75, 4 кƒж/моль.
14. ак изменитьс€ скорость пр€мой реакции: 2 —ќ(г) + ќ2(г)↔ 2 —ќ2(г) при увеличении концентрации —ќ в 3 раза?
15. онстанта скорости распада пенициллина при 36 о— равна 6Ј10-6 с-1, а при 41 о— она составл€ет 1,2Ј10-5 с-1. ¬ычислите температурный коэффициент реакции.
16. ∆енщина, Ђсоблюдающа€ фигуруї, съела вне плана в составе торта 180 г глюкозы. —читать, что глюкоза полностью окисл€етс€ в организме по уравнению:
—6Ќ12ќ6(к) + 6ќ2(г) = 6—ќ2(г) + 6Ќ2ќ(ж)
акое количество энергии получит организм пациентки? акое врем€ пациентка должна затратить на ручную стирку бель€ или окучивание гр€док (расход энергии 543 кƒж/ч), чтобы компенсировать излишества?
17. ћужчина, Ђслед€щий за фигуройї, выпил на вечеринке водки в пересчете на абсолютный спирт 46 г этанола —2Ќ5ќЌ. —читать, что этанол полностью окисл€етс€ в организме по уравнению: —2Ќ5ќЌ(ж) + 3ќ2(г) = 2—ќ2(г) + 3Ќ2ќ(ж)
|
|
|
акое количество энергии получит организм пациента? акое врем€ мужчина должен бегать трусцой (расход энергии 920 кƒж/ч), чтобы компенсировать излишества?
18. »нспектор √»Ѕƒƒ остановил водител€ и по его внешнему виду заподозрил, что тот находитс€ в состо€нии алкогольного опь€нени€. јнализ, проведенный в токсикологической клинике, показал, что содержание этилового спирта в крови водител€ превышает допустимую норму. ќднако водитель утверждает, что алкогольных напитков не употребл€л в течение нескольких дней. ѕри выполнении профессиональных об€занностей ему приходитс€ много времени проводить в услови€х повышенной концентрации выхлопных газов и он считает, что в его организме спирт мог образоватьс€ из CO2 и H2O. –ассчитать DGºреакции. ћожет ли происходить в организме человека самопроизвольное образование C2H5OH из CO2 и H2O?
19. „ем можно объ€снить, что при стандартных услови€х, невозможна экзотермическа€ реакци€: —ќ2 (г)+Ќ2 (г)↔ —ќ(г)+Ќ2ќ (ж)? –ассчитайте ΔG данной реакции. ѕри каких температурах данна€ реакци€ становитс€ самопроизвольной?
20. –ассчитайте тепловой эффект реакции горени€ сероводорода.
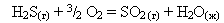
¬озможно ли самопроизвольное протекании данного процесса при стандартных услови€х?
21. ¬ лаборатории создали новый лекарственный препарат. —рок годности этого препарата при tº = 20º— составл€ет три года. »звестно, что дл€ данной реакции температурный коэффициент скорости реакции равен 2. акое врем€ можно хранить препарат при 30º—, 40º—, 50º—? ћожно ли проводить исследовани€ при температурах 100-200º—?
22. јтмосферные загр€знени€ постепенно уничтожают защитный озоновый слой «емли. ќзоновому слою угрожают поступающие в атмосферу фторированные и хлорированные углеводороды - фреоны, например, CCl3F, CCl2F2, CClF3. ќни химически стабильны в нижних сло€х атмосферы, но в стратосфере под действием ультрафиолетового излучени€ —олнца разрушаютс€, выдел€€ атомарный хлор, после чего начинают протекать реакции взаимодействи€ атомарного хлора с озоном. –ассчитайте скорость такой реакции с образованием кислорода и монооксида хлора, если через 15 сек после начала реакции мол€рна€ концентраци€ озона была 0,3 моль/л, а через 35 сек (от начала реакции) стала равна 0,15 моль/л.
23. ƒиоксид серы (SO2) Ц самый распространенный загр€знитель воздуха. ќн опасен дл€ здоровь€ людей, особенно тех, кто страдает заболевани€ми дыхательных путей. ƒиоксид серы снижает продуктивность сельскохоз€йственных культур, замедл€ет рост леса, пагубно действует на строительные материалы, содержащие карбонат кальци€. ¬ атмосфере диоксид серы окисл€етс€ до SO3; при этом роль катализатора играет наход€ща€с€ в воздухе пыль оксидов металлов. апли влаги превращают SO3 в серную кислоту, котора€ вместе с атмосферными осадками выпадает в виде "кислотных дождей". –ассчитайте значение константы скорости реакции диоксида серы с атомарным кислородом, если при концентраци€х — (SO2) и — (O), равных соответственно 0,25 и 0,6 моль/л, скорость реакции равна 0,003 моль/(лЈс).
24. ‘ерментативна€ реакци€ дегидрировани€ пропанола-2 протекает по уравнению: —Ќз—ЌќЌ—Ќз (р-р) Ѓ —Ќз—ќ—Ќз (р-р) + Ќ2(г). ќпределите тепловой эффект этой реакции по теплотам сгорани€: DЌ0сгор (—Ќ3—ЌќЌ—Ќ3) = -2002 кƒж/моль; DЌ0сгор (—Ќ3—ќ—Ќ3) = -1789 кƒж/моль; DЌ0сгор(Ќ2) = -285 кƒж/моль.
25. ¬ычислите энергию √иббса тепловой денатурации трипсина при 500—, если при 250— ∆Ќореак = 283 кƒж/моль, а ∆Sообр, 298 = 288 ƒж/(моль∙ ). —читать, что изменение энтальпии и энтропии не завис€т от температуры в данном диапазоне.
|
|
|
–ешение контрольного задани€:
________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________ «адани€ дл€ подготовки к тесту
1. «нать основные термодинамические пон€ти€ и пон€ти€ химической кинетики
2. ƒл€ увеличени€ скорости пр€мой реакции 2 CO2(г) + O2(г)Ђ2 CO2(г) + Q необходимо:
а) повысить давление;
б) повысить температуру;
в) повысить концентрацию исходных веществ;
г) понизить давление;
д) понизить температуру;
е) повысить концентрацию продуктов реакции.
3. онстанта скорости реакции (CH3CO)2O(ж) +Ќ2ќ(ж) = 2 —Ќ3—ќќЌ(ж) при 15 º— равна 0,0454 мин -1. »сходна€ концентраци€ уксусного ангидрида равна 0,5 моль/л. „ему будет равна скорость пр€мой реакции, когда концентраци€ уксусной кислоты станет равной 0,1 моль/л.
††††††††††††††† 4. –ассчитать энтальпию образовани€ реакции 3 H2(г) + N2(г)Ђ2 NH3(г), при следующих стандартных значени€х: D H ∞298,обр(NH3(г)) = Ц 46,2кƒж/моль.






