Mashtakova K.H., Ahmadiyarova Z.K., Sertaeva Z.O.
1. Objective: Conduct copper plating of parts of the copper plate in the laboratory and prove the validity of Faraday's law experimentally.
2. Summary: Copper plating Ц process copper plating layer thickness of 1 micron to 300 microns or more. Copper coatings have excellent adhesion to various metals, high ductility and conductivity. Copper coatings are easily oxidized by atmospheric conditions and are covered by oxide patina, getting brighter stains and spots of different shades. Currently, the electroplating process as a primary or an acid copper plating is one of the most common metal coating deposition processes. This coating is widely used to protect against corrosion of parts in chemical engineering, for decorative purposes, for leveling the surface or to impart the final coating of high reflective properties.
Electric current in electrolytes
Substance whose molecules dissociate into ions in solution or in molten salts called electrolytes. This process is called electrolytic dissociation. Electric current in the electrolyte is moving ions of both signs in opposite directions. Passage of current through the electrolyte constant associated with the transfer agent and accompanied by the release of components of these materials on the electrodes. That process is called electrolysis.
If you enter into the electrolyte two electrodes (metal or coal), connected to the poles of a DC voltage source, and create a constant external electric field, then under the influence of electrical forces ions in the solution will come in the direction of move. Positive ions move to the negative electrode (cathode) and negative ions Ц a positive electrode (anode). Reaching the electrodes, the ions are discharged: the anode anions give their excess electrons, cations are reduced at the cathode. For example, a molecule of copper sulphate CuSO4 dissociate when dissolved in the Cu+ positive ions and negative ions SO4-. In addition Cu+ ions and SO4- solution also contains hydrogen (H+) and hydroxyl (OH-) ions of the water. Copper ions Cu+ discharged more easily than the hydrogen ions H+, therefore at the cathode by passing the current allocation of copper will occur:
Cu++ + 2e = —u.
SO4- Ions discharged difficult than the ions OH-. Therefore, when the current passes from the anode discharge of hydroxyl ions and oxygen is released. SO4- ions with H+ ions at the anode to form sulfuric acid. The process is different if the anode is made of copper. In this case, the discharge occurs only ions at the cathode. At the opposite anode, metal ions move in a solution. This can be explained by the fact that copper Cu atoms lose electrons easily than OH- ions, in this case, instead of oxygen evolution will occur at the transition from the anode solution Cu++ ions, i.e. Cu Ц 2e = Cu++. Consequently, electrolysis with a copper anode CuSO4 reduced to copper migration from the anode to the cathode. At the same time the amount of copper sulfate will remain unchanged in the solution
|
|
|
| Electric current in liquids | ||||
| Environment | Free carriers of electric charges | Experimental confirmation | Law | Explanations |
| Electrolytes | positive and negative ions | 
| Electrolysis (Faraday's law)
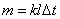 mass of substance liberated at an electrode
mass of substance liberated at an electrode
| k - electrochemical equivalent
 k - the mass -to- charge ratio
k - the mass -to- charge ratio
|
We derive Faraday's law, based on our knowledge
Mass evolved at the cathode substance is:
m = moiNi,
where mi Ц the ion mass, Ni Ц number of ions.
moi =  ,
,
where M Ц molar mass, NA Ц Avogadro's number.
Ni =  ,
,
where  Ц ion charge, and qoi = ne, n Ц valence.
Ц ion charge, and qoi = ne, n Ц valence.




The electrochemical equivalent is the ratio of the mass of the ion to its charge. We recorded Faraday's law and electrochemical equivalent. For each substance, a different electrochemical equivalent.
The laws of electrolysis
Let the charge of an ion is Ze, where e Ц elementary charge, the Z Ц valence of the ion, i.e., the number of electrons is given or acquired in the dissociation of each atom. The charge, to give the electrode is equal to:
q = n ∙ Z ∙ e (1)
where n Ц number of ions.
On the other hand, the mass M evolved on the electrode material is equal to:
ћ = n ∙ m (2)
where m Ц mass of the ion.
From the formulas (1) and (2) we find:
ћ =  ∙ q (3)
∙ q (3)
It is known that one mole of any substance ν contains the same number of atoms: N = NA; NA = 6.023Ј10 mole (Avogadro's number). Then the ion mass (mass of two electrons from an atom torn neglect) will be equal to:
m = 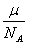 (4)
(4)
where μ Ц molar mass.
Substituting equation (4) into (3), and obtain:
ћ =  (5)
(5)
The value of the constant for each substance, called the electrochemical equivalent of the substance:
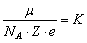 (6)
(6)
Thus, the mass M of the extracted substance on the electrode is proportional to the charge of q, passed through the electrolyte, i.e., the value of current (Faraday's first law).
Advantages and disadvantages of copper plating electrolytes.
| Copper plating electrolytes are divided into | |||
| Acidic | Alkaline | ||
| fluoroborate and sulfuric acid as the electrolyte | cyanide electrolyte-pyrophosphate | ||
| Advantages | Disadvantages | Advantages | Disadvantages |
| Ease of composition; Stability, high output current (100%) | Impossibility of direct zinc coating and the steel parts due to the contact separation of copper having poor adhesion to the base metal; A slight scattering power and a rough structure of precipitation | High throwing power; Fine-grained structure of precipitation | Low current density; Instability of the composition due to the carbonation of free cyanide by the air of carbon dioxide; Low current efficiency (not more than 60-70%) |
4. Options:
Ц room temperature;
Ц time (5 min);
Ц current was varied (0.5 а; 1.0 а; 1.5 a);
Ц an electrolyte (solution).
5. Reagents and Equipment:
Ц —uSO₄*5Ќ2ќ Ц 150 g/l;
Ц H₂SO₄- 50 g/l;
Ц copper electrode;
Ц copper plate (S = 9 cm2);
Ц 500 ml flask;
Ц distilled water;
Ц mount (end sleeves);
Ц copper wire;
Ц power supply;
Ц electrolytic cell;
Ц lacquer;
Ц a tripod.
6. Progress of the work:
|
|
|
1. Prepare the electrolyte, i.e., solution. Take 75 g CuSO₄ and diluted in a glass of 500 ml of distillate. Add water and 25 g/ml H₂SO₄. Mix solution until complete dissolution of the precipitate.
2. Putting the chain. At the cathode: copper products.
At the anode: —u.
3. Treat the product with sandpaper, isolate one side of the nail, and dry them. Washed and treated with the product in 0.01 N HCl, washed in distilled water. The copper electrode is used as the cathode, dry them and weigh on the laboratory scale, record the measured weight of the M1. We perform electrolysis at different currents within 5 minutes. After completion of the electrolysis process, the product is washed with water and dried. Then the obtained product is weighed on an analytical balance. Write the value of M2. The difference M2 - M1 increments cathode mass M.







