55–60. Рассчитайте объем кислорода, выделяющегося при фотосинтезе, и объем поглощенного углекислого газа, если дан привес биомассы при фотосинтезе и предполагается (упрощенно), что привес биомассы обусловлен образованием глюкозы.
| № задачи | Привес биомассы на 1 га в день | %сухого вещества в биомассе |
Химическое равновесие
Вопросы для самопроверки
1. Провидите вывод выражения для константы химического равновесия на основе закона действия масс. В качестве примера используйте гомогенный N2+3H2=2NH3и гетерогенный процессы С+СО2 = 2СО.
2. Каков физический смысл константы равновесия? Какие факторы влияют на величину константы равновесия?
3. Что такое обратимый процесс и необратимый процесс с термодинамической и кинетической точек зрения.
4. Что такое смещение равновесия?
5. Какие факторы влияют, и какие не влияют на положение равновесия?
6. Сформулируйте принцип Ле-Шателье.
7. Укажите, как смещается равновесие при увеличении (уменьшении) давления, температуры, концентрации исходных веществ для реакций:
CO2+CaCO3+H2O(пар) 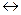 Ca(HCO3)2+Q
Ca(HCO3)2+Q
CaCO3 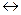 CO2 + CaO – Q
CO2 + CaO – Q
Ca(OH)2 +CO2 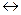 CaCO3 + H2O(пар) + Q
CaCO3 + H2O(пар) + Q
CaO+ H2O(пар) 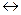 Ca(OH)2 + Q
Ca(OH)2 + Q
8. Как нужно изменить условия (давление, температуру, концентрацию веществ), чтобы увеличить выход аммиака в реакции 3H2+N 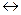 2NH3 + 98 кДж/моль?
2NH3 + 98 кДж/моль?
9. Как связана константа равновесия процесса с изменением изобарно-изотермического потенциала реакции?
Решение типовых задач
1. Вычислить равновесные концентрации водорода и йода при температуре 600°К в реакции H2 + J2=2HJ, если их начальные концентрации составляют 0,03 моль/л, а равновесная концентрация йодистого водорода равна 0,04 моль/л. Найти константу равновесия и величину изобарно-изотермического потенциала реакции при этой температуре.
Решение. На образование двух молей HJ в соответствии с уравнением реакции H2+J2 =2HJ идет один моль Н2 и один моль J2, поэтому для образования 0,04 моля HJ требуется 0,02 моля Н2 и 0,02 моля J2, отсюда их равновесные концентрации составляют 0,03–0,02 = 0,01 моля.
Для расчета константы равновесия после написания выражения в общем виде подставим в это выражение численные данные:
К=  =
= 
Далее рассчитываем изменение изобарно-изотермического потенциала этого процесса, используя связь величины ΔG и К.
ΔG = – RT In K = – 2,303 RT lg К
ΔG= – 2,303 • 8,313 · 600 • Ig 16 = – 2,303 • 8,313 – 600 ·1,204 =
= – 13830 Дж/моль = – 13,830 кДж/моль.
Итак, равновесные концентрации водорода и иода равны 0,01 моль/л, константа равновесия равна 16, величина изобарно-изотермического потенциала равна – 15,798 кДж/моль.
2. Найти константу равновесия при температуре 25 °С реакции:
С2Н2 (газ.) +2Н2 (газ.) = С2Н6 (газ.),
если ΔН°= - 324,2 кДж/моль и ΔS°= - 10,22 Дж/к·моль.
Решение. Связь между константой равновесия в стандартных условиях и стандартным изобарно-изотермическим потенциалом реакции выражается уравнением ΔG°= – RTlnK.
ΔG°= - 2,3·8,3·293·lgK (1)
Стандартный изобарный потенциал связан со стандартными значениями энтальпии и энтропии реакции соотношением:
ΔG°=ΔH°–TΔS° (2)
Найдем величину изобарно-изотермического потенциала по уравнению (2) из данных задачи, переведя ΔН° в джоули.
ΔG0 = - 324200 – 298· (- 10,22) = - 327245 Дж/моль
Теперь, пользуясь найденным значением и уравнением (1), рассчитаем lg К в кДж/моль:
lgK= - 
Используя таблицу антилогарифмов, находим значение К; К= 1,139.






