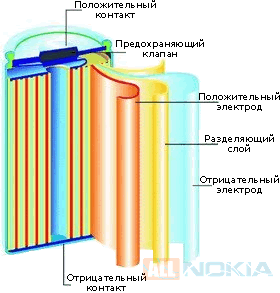
An electrochemical battery consists of cathode, anode and electrolyte. When charging the battery, the accumulation of electrons at the anode, which creates a voltage potential between the anode and the cathode. In normal operation, as power supply current flows from the cathode to the anode through the load. When charging the battery the current flows in the opposite direction. The electrodes of the battery are connected in two different ways, the first is electric circuit through which electrons flow to feed the load and the second through the electrolyte, where ions move between the electrodes through the dielectric separator (separator). The electrode that releases electrons during a redox reaction is called the anode. The electric potential of the anode of a galvanic cell is negative relative to the cathode. A chemical reaction in the battery is a reversible process, and therefore, the polarity of the electrodes changes depending on the operation mode (charge/discharge), but the terminal marking is always constant. In tables 1, 2, 3, 4 describes the structure and processes in lithium, lead, Nickel and alkaline batteries.
Тable 1. Composition and processes in Lithium-ion batteries
| Li-ion battery | Cathode | Anode | Electrolyte |
| material composition of the elements | lithium metal oxides | carbon based | lithium salts in an organic solven |
| composition and processes in charged state | a metal oxide with an intercalation structure | migration of lithium ions to the anode | |
| composition and processes in discharged state | lithium ions return to the positive electrode | mostly carbon based |
Тable 2. Composition and processes in lead-acid batteries
| Lead-acid battery | Cathode | Anode | Electrolyte |
| material composition of the elements | lead dioxide | gray spongy lead | hydrochloric acid |
| composition and processes in charged state | lead dioxide, the electrons join | lead, electrons are detached | strong sulphuric acid |
| composition and processes in discharged state | lead is converted to lead sulfide at the anode with the release of electrons, and attached at the cathode | weak sulfuric acid (diluted with water) |
Table 3. Composition and processes in NiMH and NiCd batteries.
| NiMH,NiCd | Cathode | Anode | Electrolyte |
| material composition of the elements | Ni | NiMH NiCd | potassium hydroxide |
Table 4. Composition and processes in alkaline batteries.
| Alkaline battery | Cathode | Anode | Electrolyte |
| material composition of the elements | manganese dioxide | zinc | an aqueous solution of alkali |
When submerged unpressurized system the design of the battery, a liquid electrolyte flowing between two electrodes. In hermetic designs the electrolyte is usually in the role of impregnation of the separator to provide the movement of ions from anode to cathode and back when charging. Ions are atoms, which electrons have attached or lost. They acquire the ability to move between the electrodes through the separator.