—лайд є1
Vinogradov Alexander є120
Ђ“аблица ћенделееваї
—лайд є2
1.ƒмитрий »ванович ћенделеев родилс€ 27 €нвар€ 1834 года в городе “обольске.
2.–усский учЄный, энциклопедист, химик, физик, метролог, экономист, технолог, геолог, метеоролог, нефт€ник, педагог, воздухоплаватель, приборостроитель.
3.ѕрофессор —анкт-ѕетербургского университета.
4.„лен-корреспондент »мператорской —анкт-ѕетербургской јкадемии наук.
5.—реди наиболее известных открытий Ч периодический законхимических элементов.

ѕ≈–≈¬ќƒ:
1.Dmitry Ivanovich Mendeleyev was born on January 27, 1834 in the city of Tobolsk.
2.Russian scholar, lexicographer, chemist, physicist, metrologist, economist, engineer, geologist, meteorologist, oilman, teacher, balloonist, Instrument.
3.Professor of St. Petersburg University.
4.Corresponding Member of the St. Petersburg Imperial Academy of Sciences.
5.Among the most famous discoveries - zakonhimicheskih periodic elements.
—лайд є3
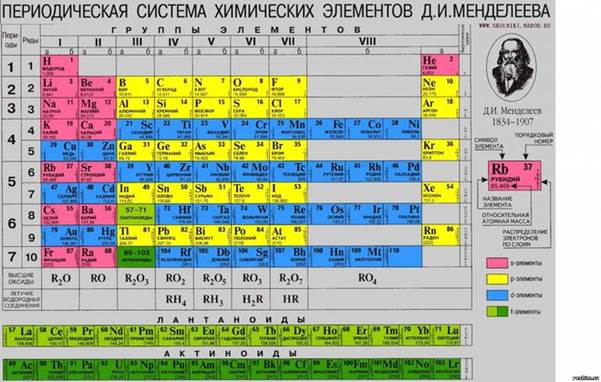
≈Є первоначальный вариант был разработан ƒ. ». ћенделеевым в 1869Ч1871 годах и устанавливал зависимость свойств элементов от атомной массы.
середине XIX века были открыты 63 химических элемента. ѕопытки найти закономерности в этом наборе предпринимались неоднократно. 
ѕервую попытку расположить элементы в пор€дке возрастани€ атомных весов предприн€л јлександр Ёмиль Ўанкуртуа (1862).
оторый разместил элементы вдоль винтовой линии и отметил частое циклическое повторение химических свойств по вертикали.
ќбе указанные модели не привлекли внимани€ научной общественности.
ѕеревод:
1.Its initial version was developed by Mendeleev in the years 1869-1871 and established the dependence of the properties of elements from the atomic mass.
2.By the middle of the XIX century 63 chemical elements were discovered. Trying to find patterns in this set have been many times.
3.The first attempt to arrange elements in order of increasing atomic weights taken Emil Chancourtois Alexander (1862).
4. Posted by members vdolvintovoy line and said part of the cyclic repetition of chemical properties of vertically.
5.Both of these models have not attracted the attention of the scientific community
—лайдє4

23 элемента открыли ученые из ¬еликобритании
ѕо 19 элементов открыли ученые из Ўвейцарии и √ермании
ѕо 17 элементов открыли ученые из —Ўј и ‘ранции
6 элементов открыли ученые из –оссии
ѕо 2 элемента открыли ученые из јвстрии »спании и д.р.
ѕо 1 элементу открыли ученые из ‘инл€ндии »талии и д. р.
ѕеревод:
23 elements discovered by scientists from the UK
On 19 elements discovered by scientists from Switzerland and Germany
On 17 elements discovered by scientists from the US and France
6 elements discovered by scientists from Russia
On 2 element discovered by scientists from Austria, Spain and other
On 1 element discovered by scientists from Finland, Italy and other
—лайдє5
|
|
|

1.ѕо легенде, мысль о системе химических элементов пришла к ћенделееву во сне.
2. ќƒЌј ќ »«¬≈—“Ќќ „“ќ Ё“ќ Ќ≈ “ј
3.ќднажды на вопрос, как он открыл периодическую систему, учЄный ответил: Ђя над ней, может быть, двадцать лет думал, а вы думаете: сидел и вдругЕ готової.
4. —ущность открыти€ ћенделеева заключалась в том, что с ростом атомной массы химических элементов их свойства мен€ютс€ не монотонно, а периодически.
5. Ќаиболее распространЄнными €вл€ютс€ 3 формы таблицы ћенделеева: Ђкоротка€ї Ђдлинна€ї и Ђсверхдлинна€ї.
ѕеревод:
According to legend, the idea of a system of chemical elements in Mendeleev came in a dream.
However, we know that it is not
Once the question of how he discovered the periodic system, the scientist replied, "I'm over it, maybe twenty years of thought, as you think, and suddenly sat... ready."
The essence of Mendeleev's discovery was the fact that with increasing atomic weight of chemical elements change their properties are not monotone, and periodically.
The most common are 3 forms of the periodic table, "short", "long" and "super-long."
—лайд є6

ƒальнейшее развитие периодической системы св€зано с заполнением пустых клеток таблицы.
¬ которые помещались всЄ новые и новые элементы.
Ёто благородные газы, природные и искусственно полученные радиоактивные элементы.
¬2010 году, с синтезом 117 элемента, седьмой период периодической системы был завершЄн.
ѕроблема нижней границы таблицы ћенделеева остаЄтс€ одной из важнейших в современной теоретической химии.
ѕеревод:
Further development of the periodic system associated with the filling of the empty table cells.






