Исходное применение паклитаксела улучшает результат по сравнению с комбинированной терапией схемой CMFP в качестве терапии первой линии при нелеченом метастатическом раке молочной железы
Дж.Ф. Бишоп, Дж. Девар, Г.К. Танер, Дж. Смит, М.Х.Н. Таттерсол, Я.Н. Оливер, С. Окланд, Я. Кеннеди, Д. Голдштейн, Г. Гарни, Е. Вайпоул, Д. Леви, Дж. Стефенсон и Р. Канетта от имени Группы по исследованию Таксола, Австралия/Новая Зеландия
Цель исследования.
Определить место паклитаксела, используемого в качестве монотерапии, по сравнению с неантрациклиновой комбинированной химиотерапией в качестве лечения первой линии при метастатическом раке молочной железы.
Больные и методы.
Больных с ранее нелеченым метастатическим раком молочной железы рандомизировали на получение или паклитаксела в дозе 200 мг/м2внутривенно (в/в) на протяжении 3 часов в течение восьми курсов (24 недели), или на стандартную схему, включавшую циклофосфамид (100 мг/м2/день перорально в дни с 1-го по 14-й), метотрексат (40 мг/м2 в/в в дни 1-й и 8-й), фтороурацил (600 мг/м2 в/в в дни 1-й и 8-1) и преднизон (40 мг/м2/день перорально в дни с 1-го по 14-й) (схема CMFP) на протяжении шести курсов (24 недели); эпирубицин был рекомендован в качестве терапии второй линии.
Результаты.
Всего было рандомизировано 209 удовлетворяющих критериям участия в испытании больных, медианная продолжительность выживания составляла 17,3 месяца для паклитаксела и 13,9 месяца для CMFP. Анализ с использованием нескольких переменных показал, что больные, получавшие паклитаксел, жили значительно дольше, чем больные, получавшие схему CMFP (Р =0,025). Паклитаксел вызывал достоверно меньше тяжелой нейтропении/тромбоцитопии, мукозитов, документированных инфекций (для всех Р <0,001), тошноты или рвоты (Р =0,003) и лихорадки без документированной инфекции (Р =0,007), а также меньше случаев фебрильной нейтропении по сравнению со схемой CMFP (Р =0,001). Алопеция, периферическая нейропатия, а также миалгия или артралгия были более тяжелыми при использовании паклитаксела (для всех Р <0,0001). В целом, качество жизни было одинаковым для обеих схем (Р ≥0,07).
Вывод.
Исходное применение паклитаксела давало достоверно меньшую миелосупрессию и меньшее число инфекций при более длительной выживаемости и аналогичном качестве жизни и контроле метастатического рака молочной железы по сравнению со схемой CMFP.
* * *
В Соединенных Штатах ежегодно диагноз рака молочной железы ставится более чем 180 000 женщин и более 45 000 женщин умирает от этого заболевания [1]. Несмотря на серьезные успехи в области адъювантной терапии, метастатический рак молочной железы остается серьезной клинической проблемой, затрагивающей большое число больных. На протяжении многих лет стандартные комбинации химиотерапии были основным направлением лечения форм метастатического заболевания, которые резистентны к гормонам, имеют негативный статус по эстрогеновым рецепторам, угрожают жизни больной или поражают висцеральные органы. Схемы исходной химиотерапии представляли собой или комбинации циклофосфамид, метотрексата, фтороурацила и преднизона (CMFP), после которых следовало применение антрациклина, или комбинации, содержащие доксорубицин [2-4]. Выбор адекватной исходной химиотерапии часто ограничивается ранними рецидивами у больных, которые недавно получали адъювантную химиотерапию с использованием тех же комбинаций, кроме того такой выбор ограничивается состоянием больной. Поэтому важно определить место новых противоопухолевых препаратов в терапии рака молочной железы. В то время, когда они были впервые описаны [5], комбинации типа CMFP, по имевшимся сообщениям, давали высокий процент ответа на лечение. При оценке с использованием современных критериев и все более совершенных методов объективной визуализации схема CMFP с добавлением винкристина или без него дает объективные ответы у 37-68% больных, а медианная продолжительность ответа варьирует от 6 до 11 месяцев. Медианная выживаемость, считая с момента начала лечения, составляет от 7 до 16 месяцев [6-15]. Доксорубицин в форме монотерапии столь же активен, как и комбинации CMFP по данным рандомизированных испытаний распространенного рака молочной железы [2, 8, 16]. В этих испытаниях доксорубицин давал меньшую продолжительность ответа при использовании в виде индивидуального агента, однако четких различий в выживаемости не наблюдалось. Выживаемость в этих исследованиях трудно интерпретировать, поскольку при прогрессии заболевания больных обычно переводили на альтернативную схему лечения. Из шести рандомизированных испытаний по сравнению комбинаций циклофосфамида, доксорубицина и фтороурацила с комбинациями CMFP при распространенном раке молочной железы в трех был продемонстрирован достоверно более высокий процент ответа при использовании комбинаций циклофосфамида, доксорубицина и фтороурацила [17-23]. Однако только в двух из шести исследований было показано статистически достоверное преимущество комбинаций, содержащих доксорубицин, в отношении выживаемости. Эти исследования были интерпретированы как демонстрация небольшого преимущества комбинаций с доксорубицином при метастатическом раке молочной железы [17]. Недавно проведенный мета-анализ испытаний по химиотерапии с метастатическим раком молочной железы был посвящен рассмотрению испытаний, в которых сравнивались схемы, содержащие антрациклин, со схемами без антрациклина [24]. Комбинации, содержащие антрациклин, давали более высокие проценты ответа, однако общая выживаемость была одинаковой. Схема CMFP использовалась как терапия первой линии при метастатическом раке молочной железы, после раннего рецидива при адъювантной терапии на основе антрациклинов, а также у инвалидизированных больных, где целью лечения является смягчение симптоматики, или же по выбору больной в связи с побочными эффектами. В Австралийском рандомизированном испытании комбинацию доксорубицина и циклофосфамида сопоставляли со схемой CMFP у 305 ранее не леченых больных с метастатическим раком молочной железы [12]. Ответ, продолжительность ответа и выживаемость были сходными для схемы CMFP и комбинациями доксорубицин/циклофосфамид, применявшимися непрерывно до рецидива. Использование комбинаций с доксорубицином приводило к достоверно большему числу случаев тошноты, рвоты и алопеции по сравнению со схемой CMFP. Этот опыт явился дополнительным аргументом в пользу использования схемы CMFP в качестве контрольной группы для нашего рандомизированного испытания схемы CMFP по фазе III в сравнении с монотерапией паклитакселом. CMFP является широко применяемой комбинацией как при адъювантной терапии, так и при лечении распространенной формы опухоли, особенно для больных с хорошим прогнозом. Таким образом, результаты этого сравнения могут иметь более широкое значение. Паклитаксел представляет собой новый цитотоксический агент, связывающийся с мономером бета-тубулина и вызывающий перманентную полимеризацию микротрубочек [25, 26]. Потеря динамической реорганизации микротрубочек во время митоза приводит к селективной блокаде на фазе G2/M клеточного деления. Этот эффект in vitro коррелирует с клинической активностью [27]. Паклитаксел, назначаемый в форме 24-часового вливания, активен у ранее не леченых больных с распространенной формой рака молочной железы [28-33]. Продолжительность вливания паклитаксела может быть различной, в других исследованиях сообщают о 3- часовом вливании или, что было уже после начала нашего испытания, об одночасовых вливаниях [34-37]. Крупномасштабное рандомизированное Европейско-Канадское испытание было посвящено сравнению двух доз паклитаксела (135 мг/м2 и 175 мг/м2) и двух сроков вливания (3 часа и 24 часа) при рецидиве рака яичников. Для двух доз и для двух скоростей вливания различий в результате не было. Частота реакций гиперчувствительности в этом испытании была низкой, и на нее влияли доза или время введения. Однако в этом исследовании было четко показано, что 24-часовое вливание паклитаксела дает достоверно большее снижение числа нейтрофилов после каждого курса по сравнению с 3-часовым вливанием [34]. Это наблюдение было подтверждено другим недавним рандомизированным испытанием по сравнению вливания в течение 3, 6 и 24 часов [38]. На основании этих данных и на основании удобства проведения для настоящего испытания было выбрано трехчасовое вливание. В последующем рандомизированное испытание по фазе III по сравнению 3-часового и 96-часового вливания паклитаксела продемонстрировало отсутствие отличий при метастатическом раке молочной железы [39]. На момент начала настоящего испытания было неясно, важна ли доза паклитаксела при лечении метастатического рака молочной железы. Однако в ранних исследованиях сообщалось о высоком проценте ответа при дозах паклитаксела 200 мг/м2 и 250 мг/м2, когда препарат вводился в форме 24-часового вливания, что послужило основанием для выбора дозы паклитаксела в 200 мг/м2 для настоящего испытания [40, 41]. В дальнейшем Nabholtz с соавт. [42] высказали предположение, что доза паклитаксела, равная 175 мг/м2, может увеличивать время до прогрессии по сравнению с дозой 135 мг/м2.
БОЛЬНЫЕ И МЕТОДЫ
Больные
Критериями участия в испытании были гистологически подтвержденный метастатический или местно-распространенный рако молочной железы (стадия III или IV) или рецидив рака молочной железы после хирургической операции, ранее проведенной лучевой терапии (более, чем за 4 недели), или после ранее проводившейся адъювантной химиотерапии (более чем за 6 месяцев), однако при отсутствии ранее проводившейся химиотерапии по поводу распространенного заболевания. Требования к больным включали функциональный статус по ECOG от 0 до 2; наличие измеримой опухоли или опухоли, которую можно было оценить в плане ответа на лечение; адекватная функция костного мозга, печени и почек; ожидаемая продолжительность жизни не менее 3 месяцев; и полученное после информирования письменное согласие на участие в испытании. Больные не удовлетворяли критериям для участия в испытании, если у них, помимо рака молочной железы, ранее или в настоящее время имелось другое злокачественное заболевание, за исключением рака кожи (немеланомного характера) или карциномы шейки матки in situ; другими состояниями, исключающими участие в испытании, были кардиоаритмии в истории болезни, застойная сердечная недостаточность, а также документированный инфаркт миокарда, перенесенный в предыдущие 6 месяцев, нейропатия степени 2 или более тяжелая по по критериям ВОЗ, подтвержденный метастаз в мозг в качестве единственного проявления метастазирования, слабоумие или измененный психический статус, не дающий возможности на получение информированного согласия [43].
Вопросы этики
Протокол был утвержден комитетами по этике каждого из участвовавших в испытании центров. От каждого больного после информирования было получено письменное согласие на участие. О всех серьезных побочных эффектах сообщали спонсору испытания, а также комитетам по этике соответствующих учреждений согласно их требованиям об отчетности. Испытание было спланировано, проведено, проанализировано и описано в соответствии с правилами качественной практики клинических исследований в Австралии [44].
Критерии токсичности и ответа на лечение
Использовались стандартные критерии ВОЗ для оценки токсичности и ответа на лечение [43]. Качество жизни оценивали с использованием линейных аналоговых шкал, заполняемых каждой больной, и индекса качества жизни по Spitzer, который заполнялся врачом [12, 45].
Лечение
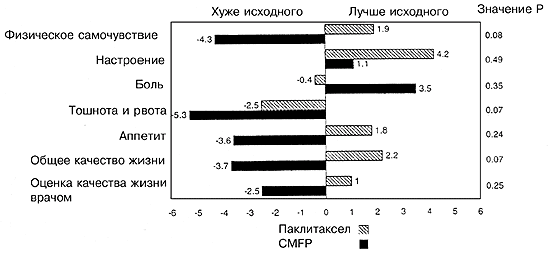
Больные подвергались стратификации по учреждению и рандомизировались или на внутривенное (в/в) введение 200 мг/м2 паклитаксела (Таксол, Бристол-Майерс Сквибб, Принстон, Нью-Джерси) на протяжении 3 часов с интервалом в 21 день в течение восьми курсов (24 недели), или на получение циклофосфамида (100 мг/м2/день перорально в дни с 1-го по 14-й), метотрексат (40 мг/м2 в/в в дни 1-й и 8-й), фтороурацил (600 мг/м2 в/в в дни 1-й и 8-1) и преднизон (40 мг/м2/день перорально в дни с 1-го по 14-й с интервалом в 28 дней на протяжении шести курсов (24 недели) (Рис. 1). Больным, получавшим паклитаксел, проводилась премедикация дексаметазоном в дозе 20 мг перорально за 12 и 6 часов до химиотерапии. Дифенгидрамин (димедрол) в дозе 50 мг или прометазин (25 мг) и циметидин (300 мг в/в) или ранитидин (50 мг в/в) вводили за 30 минут до химиотерапии. Антиэметики вводились по усмотрению врача-исследователя. Больным с прогрессией заболевания во время получения терапии первой линии рекомендовалось введение эпирубицина в дозе 90 мг/м2 (в/в) каждые 3 недели. После 6 месяцев исходной химиотерапии больные со стабильным заболеванием или объективным ответом наблюдались в отсутствие лечения вплоть до рецидива.
Модификация дозы
Модификации дозы основывались на значении минимального содержания форменных элементов крови, причем снижение дозы на 25% проводилось при абсолютном содержании нейтрофилов менее 0,5 х 109/л, содержании тромбоцитов менее 50 х 109/л и/или при фебрильной нейтропении (лихорадка >38°С при абсолютном содержании нейтрофилов менее 0,5 х 109/л) или при значительном кровотечении, связанном с тромбоцитопенией. Больным с абсолютным содержанием нейтрофилов менее 0,5 х 109/л или с содержанием тромбоцитов менее 100 х 109/л на запланированные даты проведения курсов лечения назначалась недельная задержка до восстановления при корректировке дозы, основанной на содержании форменных элементов крови в надире. При любой негематологической токсичности степени 3 (за исключением алопеции и тошноты/рвоты) проводилось снижение дозы на 25%. Любая больная с негематологической токсичностью степени 4 по ВОЗ должна была сниматься с испытания. Больные с симптоматической аритмией или атриовентрикулярной блокадой должны были прекратить лечение. Больным с реакциями гиперчувствительности при наличии гипотензии или ангионевротического отека, дыхательной недостаточности или генерализованной крапивницы вливание паклитаксела останавливали и принимали меры в отношении реакции гиперчувствительности.
Обследования
Запланированные обследования включали полный анализ крови, который выполнялся до лечения и с недельными интервалами в последующем. Анализ мочевины, электролитов, функциональные тесты печени, оценку токсичности по ВОЗ, физическое обследование и оценку качества жизни осуществляли до лечения и в дальнейшем при каждом курсе лечения. Обследования для выявления и мониторинга метастатического заболевания включали КТ-сканирование, сканирование костного скелета и рентгенографию до проведения лечения, анализы повторяли через 12 недель и через 24 недели лечения. Эти анализы также повторяли во время предполагаемого рецидива или прогрессии, и с интервалами не менее 4 недель при подтверждении частичного или полного ответа на лечение. После завершения лечения больных наблюдали ежемесячно до рецидива, а в последующем их статус исследовали с 3-месячными интервалами до их смерти или анализа материалов исследования.
Статистические методы
Запланированное число больных для участия в испытании составляло 200 человек. Больные стратифицировались каждым из участвующих в испытании центров до проведения рандомизации. Созданные с помощью компьютера схемы рандомизации были подтовлены для каждого центра и находились в статистическом центре Онкологического института Питер МакКаллум (Мельбурн, Австралия). Рандомизация была основана на процедуре случайного выбора из двух вариантов с адаптивной коррекцией, причем в случае каждого выбора адаптивный коэффициент равнялся 3n в пользу группы с числом больных, меньшим на n, чем другая. В каждом центре все данные собирались специалистами по данным, проверялись наблюдателями компаний- спонсоров, а также центра, проводящего испытание, и вводились в базу данных в статистическом центре. Один запланированный промежуточный анализ проводился после того, как в каждую группу было набрано по 23 больных, чтобы проверить, что процент ответа составлял по крайней мере 25% для каждой из групп лечения. Полученные результаты не были сообщены врачам-исследователям. Второй промежуточный анализ был проведен на основании первых 100 больных для представления на конференции, однако это было уже после завершения набора всех больных в испытание. Поэтому результаты этого анализа, не могли повлиять ни на набор больных, ни на интерпретацию окончательных результатов испытания. Результаты испытания (основные конечные результаты) приводятся по отношению к полному числу включенных в испытание больных. Процент ответа для двух групп сравнивался с использованием двустороннего точного критерия Фишера, используя пакет StatXact 3 для Windows (Cytel Software Corp., Кембридж, Массачузетс). Процент больных без прогрессии, а также общую выживаемость оценивали в соответствии с методом Каплана-Мейера, используя пакет S-PLUS, версия 3.3 для Windows (StatSci, Сиэттл, Вашингтон). Время отсчитывали, начиная от даты рандомизации, а заключительной датой для всех анализов на выживаемость было 20 февраля 1997 г. Все летальные исходы зачитывались как события при общем анализе на выживаемость и как прогрессия заболевания и как летальный исход без прогрессии для кривых выживаемости без прогрессии заболевания. Для оценки 95%-ных доверительных интервалов для медианных значений выживаемости использовали метод Брукмейера- Кроули. Различия между группами анализировались с использованием лог-рангового критерия Мантел-Кокса (пакет S-PLUS, версия 3.3 для Windows). Категории токсичности сравнивали для двух групп рандомизации с использованием критерия Кохрана-Армитаджа для линейных трендов с точным интерполированием, двусторонним, если не указано по-иному (пакет StatXact 3 для Windows). Локализацию заболевания классифицировали по следующим категориям: кожа и мягкие ткани, кости, печень, легкие (включая выпот в плевру) и прочие висцеральные локализации (включая мозг). Тест на прогностические факторы проводили при однофакторном анализе ответа на лечение, выживаемости без прогрессии и общей выживаемости. Такие факторы включали функциональный статус по ECOG, объем заболевания, менопаузальный статус на момент диагноза, время после постановки диагноза, ранее проводившуюся адъювантную химиотерапию, ранее проводившуюся лучевую терапию и ранее проводившуюся гормонотерапию. Многофакторный анализ проводили в отношении вышеназванных факторов плюс группы рандомизации с использованием логистической регрессии для анализа ответа (пакет LogXact для Windows, Cytel) и с использованием модели пропорциональных рисков Кокса для анализа выживаемости (SPSS Advanced Statistics 7.5, SPSS Inc., Чикаго, Иллинойс). Качество жизни оценивали перед началом лечения и после каждого курса. Использованные для оценки качества жизни инструменты были ранее описаны в литературе и верифицированы многими исследователями, участвовавшими в настоящем испытании. При оценке больная делала отметку на каждой из шести линейных аналоговых шкалах (физическое самочувствие, настроение, боль, тошнота/рвота, аппетит, общая оценка качества жизни), а врачи заполняли индекс качества жизни по Спицеру, состоявший из пяти вопросов с ответами, имевшими значения балла от 1 до 3. Критерий ранговых сумм по Вилкоксону использовался для сравнения групп лечения в отношении среднего улучшения для каждой характеристики качества жизни во время периода лечения по отношению к исходным значениям [46].
РЕЗУЛЬТАТЫ
В 17 центрах, участвовавших в испытании в Австралии и в Новой Зеландии, было набрано 209 больных: 107 из них было рандомизировано на получение паклитаксела, а 102 на лечение схемой CMFP. Медианный возраст больных составлял 54 года для обеих групп (интервал для паклитаксела – от 36 до 73 лет, для CMFP – от 32 до 80 лет). Характеристики больных на момент рандомизации приведены в Таблице 1. На момент начала участия в испытании 31% больных в группе паклитаксела и 40% больных в группе CMFP имели функциональный статус по ECOG, равный 0. Только 21% больных в группе паклитаксела и 33% больных в группе CMFP получали предшествующую адъювантную химиотерапию. Пять больных завершили адъювантную химиотерапию за 9-12 месяцев, а остальные более, чем за 12 месяцев, до рандомизации. Семьдесят два процента больных, получавших паклитаксел, и 77% больных, получавших CMFP, получали ранее эндокринную терапию. Испытание было открыто для набора больных в сентябре 1993 г., а медианное время наблюдения для больных на момент закрытия испытания составляло 26 месяцев (интервал от 17 до 40 месяцев). Две больные в группе паклитаксела и три больных в группе CMFP не получили лечения, которое они должны были получать в соответствии со своей группой рандомизации. Снижение доз более, чем на 5%, имело место у 23% больных в группе паклитаксела и у 32% в группе CMFP. У 9% больных в группе паклитаксела задержка составляла ≥1 недели, аналогичная задержка имела место у 34% больных в группе CMFP. Сорок восемь процентов больных в группе паклитаксела и 52% больных в группе CMFP завершило 24 недели лечения.
Таблица 1. Характеристики больных на момент рандомизации по группам лечения
| (n=107) | (n=102) | |
| Функциональный статус по ECOG | ||
| Локализация заболевания* | ||
| Кожа/мягкие ткани | ||
| Кости | ||
| Печень | ||
| Легкие | ||
| Прочие висцеральные органы | ||
| Основная локализация заболевания | ||
| Кожа/мягкие ткани только | ||
| Кости ±мягкие ткани | ||
| Висцеральная±кости±кожа/мягкие ткани | ||
| Статус по менопаузе на момент диагноза | ||
| До менопаузы | ||
| Менопауза/после менопаузы | ||
| Неизвестно | ||
| Время после диагноза, годы | ||
| ≤3 лет | ||
| >3 лет | ||
| Статус по эстрогеновым рецепторам | ||
| Позитивный | ||
| Негативный или пограничный | ||
| Неизвестно | ||
| Статус по прогестероновым рецепторам | ||
| Позитивный | ||
| Негативный или пограничный | ||
| Неизвестно | ||
| Предшествующая адъювантная химиотерапия | ||
| Отсутствует | ||
| CMF (винкристин) (преднизолон) | ||
| CMF (доксорубицин) | ||
| Прочие | ||
| Неизвестно | ||
| Другая предшествующая терапия | ||
| Адъювантная лучевая терапия | ||
| Паллиативная лучевая терапия | ||
| Эндокринная терапия |
*У каждой больной могла быть затронута более, чем одна локализация.
Полный ответ на лечение наблюдался у 2% больных в группе паклитаксела и у 6% больных в группе CMFP. Частичный ответ отмечался у 27% больных в группе паклитаксела и 29% больных в группе CMFP. Общий процент объективного ответа для групп паклитаксела и CMFP составлял 29% (95%-ный доверительный интервал от 21% до 39%) и 35% (95%-ный доверительный интервал от 26 до 45%), соответственно (Р =0,37; Таблица 2). Стабилизация заболевания была зарегистрирована у 37% больных, получавших паклитаксел, и 32% больных, получавших CMFP. Потенциальные прогностические факторы анализировалисьс целью определить, влияют ли они на процент ответа. Только у больных, находившихся в состоянии до менопаузы при диагнозе, наблюдался достоверно более высокий процент ответа по сравнению с женщинами в состоянии менопаузы или после менопаузы (Р =0,008). Однако не наблюдалось достоверных различий между группами лечения (Р =0,44), когда была проведена коррекция на статус в отношении менопаузы с использованием модели логистической регрессии с множественными переменными. Никакой другой из исследованных факторов достоверно не влиял на процент ответа. Процент ответа у больных на схеме CMFP, которые ранее получали адъювантную схему CMFP, не отличался достоверно от соответствующей цифры для тех больных, которые такой терапии не получали (Р =0,66). Только 4% больных, получавших паклитаксел, и 3% больных, получавших схему CMFP, были живы без признаков прогрессии заболевания на момент закрытия испытания. Оцененное медианное время до прогрессии для больных на паклитакселе составляло 5,3 месяца (95%-ный ДИ от 4,1 до 5,6 месяцев), причем 15% больных оставались без признаков прогрессии через 1 год и 3% через 2 года. Оцененное медианное время до прогрессии для больных на схеме CMFP составляло 6,4 месяца (95%-ный ДИ от 5,2 до 7,8 месяцев), причем 17% больных оставались без признаков прогрессии через 1 год и 5% через 2 года. Не наблюдалось достоверных различий между группами лечения (Р =0,25; Рис. 2 и Таблица 2). Индивидуальные анализы прогностических факторов, влияющих на выживаемость без прогрессии, показали, что больные с функциональным статусом по ECOG, равным 0 (Р =0,024), или сроком последиагноза, превышающим 3 года (Р =0,002), имеют достоверно более длительное выживание без признаков прогрессии заболевания.
Таблица 2. Сводка результатов по группам лечения
| Паклитаксел (n=107) | CMFP (n=102) | Р | |||
| % | 95%-ный ДИ | % | 95%-ный ДИ | ||
| Процент ответа (полный+частичный) | 21-39 | 26-45 | 0,37 | ||
| Выживаемость без прогрессии | |||||
| Оцененная медиана (мес) | 5,3 | 4,1-5,5 | 6,4 | 5,2-7,8 | 0,25 |
| Выживаемость без прогрессии на 1 год | 8-22 | 9-24 | |||
| Выживаемость без прогрессии на 2 года | 0-8 | 1-9 | |||
| Общая выживаемость | |||||
| Оцененная медиана (мес) | 17,3 | 12,6-21,4 | 13,9 | 11,4-16,5 | 0,068 |
| Выживаемость на 1 год | 52-70 | 45-65 | |||
| Выживаемость на 2 года | 29-48 | 12-29 |
При использовании модели с множественными переменными, содержащими два этих фактора, между двумя группами лечения достоверных различий не оставалось (Р =0,23). На момент закрытия испытания в группе паклитаксела было живо 30% больных, а в группе CMFP 20%. Оцененная медианная выживаемость составляла 17,3 месяца (95%- ный ДИ от 12,6 до 21,4) для больных в группе паклитаксела, причем 61% больных были живы через 1 год и 39% через 2 года. Оцененная медианная выживаемость в группе CMFP составляла 13,9 месяцев (95%-ный ДИ от 11,4 до 16,5 месяцев), причем 55% больных были живы через 1 год и 20% через 2 года. Различие между двумя группами лечения не было статистически достоверным (Р=0,068; Рис. 3 и Таблица 2). Индивидуальный анализ факторов, связанных с испытанием, показал, что больные с функциональным статусом по ECOG равным 0 (Р =0,002), невисцеральной локализацией заболевания (Р =0,0003) или с диагнозом, поставленным более, чем за 3 года до рандомизации (Р =0,002), имели достоверно лучшую выживаемость. Например, больные с функциональным статусом по ECOG, равным 0, имели медианную выживаемость, равную 20,0 месяцев по сравнению с 12,6 месяца для больных с функциональным статусом 1 или 2. Многофакторный анализ подтвердил важность этих факторов (Таблица 3). В этой модели больные с исходной группе паклитаксела имели достоверно лучшую выживаемость по сравнению с больными в группе CMFP (Р=0,025). Не наблюдалось существенных взаимодействий между тремя важными прогностическими факторами и группой лечения.
Таблица 3. Модель пропорционального риска по Коксу для факторов, влияющих на выживаемость
| Фактор | Группа сравнения | Относительная летальность | Р | |
| ≤3 лет после диагноза | >3 лет | 2,0 | 1,4-2,8 | <0,0001 |
| Статус по ECOG 1-2 | 2,0 | 1,4-2,8 | 0,0001 | |
| Висцеральное заболевание | Не висцеральное | 2,0 | 1,3-2,9 | 0,0003 |
| Паклитаксел | CMFP | 0,7 | 0,5-1,0 | 0,025 |
Лейкопения, тромбоцитопения, тошнота и рвота, а также мукозит – все эти побочные эффекты носили достоверно менее тяжелый характер для больных в группе паклитаксела по сравнению с группой CMFP (Таблица 4). Фебрильная нейтропения и/или инфекция наблюдались у 10% больных в группе паклитаксела и 27% больных в группе CMFP (Р =0,001). Средняя продолжительность госпитализаций по поводу фебрильной нейтропении или инфекции составляла 1,5 дня для паклитаксел и 4,4 дня для CMFP (Р =0,0006). В целом 1,6% курсов паклитаксела и 8,2% курсов CMFP требовали госпитализации по поводу фебрильной нейтропении и/или документированной инфекции. Алопеция, миалгия/артралгия и периферическая нейропатия были достоверно более тяжелыми в группе паклитаксела по сравнению с группой CMFP (Р <0,0001). Периферическая нейропатия степени 3 отмечалась у 9% больных в группе паклитаксела, а у 1% больных она имела степень тяжести 4. Реакции гиперчувствиетльности во время лечения паклитакселом отмечались только у трех больных, причем эти реакции не имели никаких последствий. Качество жизни оценивали в начале участия в испытании и после каждого курса лечения. Расчитывали среднее значение качества жизни для всех курсов и вычитали его из исходного значения. Оценку качества жизни проводили как сама больная, так и врач. Большинство характеристик качества жизни (за исключением болевой симптоматики) было несколько лучшим в группе паклитаксела, чем в группе CMFP, однако эти различия не были статистически достоверными (Р >0,07 для всех характеристик; Рис. 4). Тридцать три больные, исходно лечившиеся паклитакселом,и 31 больная, исходно получавшая схему CMFP, дальнейшей химиотерапии не получали (Таблица 5). Почти одинаковое число больных в обеих группах получали терапию второй линии на основе антрациклина (43 больных в группе паклитаксела; 39 больных в группе CMFP). Объективный ответ на химиотерапию второй линии на основе антрациклина отмечался у 33% больных в группе паклитаксела и 21% в группе CMFP. У больных, исходно получавших паклитаксел, при проведении химиотерапии второй линии на основе антрациклина медиана выживаемости составляла 13,3 месяца, считая от начала химиотерапии второй линии, в то время как у больных, исходно получавших схему CMFP, медиана выживаемости составляла 5,5 месяца. Поскольку отбор больных для химиотерапии второй линии мог быть связан с искажающими факторами, мы не проводили статистического сравнения в отношении двух указанных результатов.
Таблица 4. Сравнение острой токсичности в группах лечения
| % больных с каждой степенью | ||||||
| Степень по ВОЗ | ||||||
| Лейкопения | ||||||
| Паклитаксел | <0,0001 | |||||
| CMFP | ||||||
| Нейтропения | ||||||
| Паклитаксел | 0,91 | |||||
| CMFP | ||||||
| Тромбоцитопения | ||||||
| Паклитаксел | <0,0001 | |||||
| CMFP | ||||||
| Тошнота/рвота | ||||||
| Паклитаксел | 0,0032 | |||||
| CMFP | ||||||
| Мукозит | ||||||
| Паклитаксел | ||||||
| CMFP | 0,0002 | |||||
| Периферическая нейропатия | ||||||
| Паклитаксел | ||||||
| CMFP | <0,0001 | |||||
| Миалгия/артралгия | ||||||
| Паклитаксел | ||||||
| CMFP | <0,0001 | |||||
| Алопеция | ||||||
| Паклитаксел | - | |||||
| CMFP | - | <0,0001 | ||||
| Документированная инфекция | ||||||
| Паклитаксел | 0,0006 | |||||
| CMFP | ||||||
| Лихорадка без документированной инфекции | ||||||
| Паклитаксел | 0,0069 | |||||
| CMFP |
Таблица 5. Химиотерапия второй линии*
| Исходное лечение | ||
| % паклитаксела (n=105) | % CMFP (n=99) | |
| Химиотерапия второй линии | ||
| Не проводилась | ||
| На основе антрациклина | ||
| Типа CMFP | ||
| Таксан | ||
| Прочее |
*Не приведены данные по пяти больным, не получавшим лечения после исходной рандомизации.
ОБСУЖДЕНИЕ
Наши результаты показывают, что исходное применение паклитаксела в форме монотерапии дает противоопухолевый эффект, близкий к наблюдавшемуся при использовании комбинированной схемы CMFP, причем процент объективных ответов и значения времени до прогрессии заболевания являются близкими. Процент ответа для паклитаксела сходен с цифрами, которые ранее описаны для паклитаксела в форме монотерапии, вводимого путем 3-часового вливания. Процент ответа на схему CMFP довольно низок, однако находится в интервале, ранее описанном литературе. Отметим, что 95%-ный доверительный интервал для ответа на CMFP в настоящем испытание указывает, на объективные ответы в интервале от 26 до 45%. Процент ответа может отражать использование более интенсивных и совершенных методов визуализации опухоли, используемых в современных испытаниях по онкологии, а также трудность выявления изменений в поражении костей. Еще важнее то, что медианное время до прогрессии и выживаемость на схеме CMFP близки к тем, которые ранее были описаны для этой комбинации в Австралии и в других испытаниях [2-4, 6-14]. При анализе с использованием одной переменной общая выживаемость была несколько большей в группе паклитаксела, однако различие не было статистически достоверным (Р =0,068). Рандомизированные больные стратифицировались центрами, участвовавшими в испытании, однако не в отношении каждого из семи потенциальных прогностических факторов, три из которых, как было показано, имеют достоверное воздействие на выживаемость. При коррекции на эти факторы регрессия пропорциональных рисков по Коксу показала, что больные, находящиеся в группе паклитаксела, имели лучшую выживаемость (Р =0,025). Более длительная выживаемость представлялась клинически значимой, поскольку медианная продолжительность выживания составляла 17,3 месяца для группы паклитаксела и 13,9 месяцев для CMFP, а относительная летальность (паклитаксел/CMFP) составляла 0,70 после коррекции на прогностические факторы. Такое улучшение выживаемости является ценным для больных, поскольку на срок в 2 года улучшение составляло 19%. Однако поскольку исходный процент подавления опухоли был одинаковым, лучшая выживаемость может отражать более оптимальную последовательность лечения при условии когда лечение начинали с применения паклитаксела. Анализ форменных элементов крови проводился еженедельно, чтобы иметь точную картину относительной миелосупрессии в двух группах. Паклитаксел вызывал достоверно меньшую лейкопению и тромбоцитопению. Нейтропения была близкой для двух групп, в то время как число случаев инфекций, госпитализаций по поводу инфекций и продолжительность госпитализаций были значительно меньшими в группе паклитаксела, чем в группе CMFP. Возможное объяснение заключается в том, что в группе CMFP значительно больше случаев мукозита, а мукозит дает точку входа инфекции в организм. Кроме того, продолжительность нейтропении в случае паклитаксела была значительно меньшей, о чем говорит число больных, для которых требовалась отсрочка лечения. Алопеция, миалгия и периферическая нейропатия были достоверно более выраженными в группе паклитаксела. Что касается периферической нейропати, она имела степень 3 или 4 только у 10% больных. В целом, качество жизни в обеих группах было приемлемым для больных и не слишком сильно отличалось для двух групп лечения. Больных в обеих группах просили отмечать общее качество жизни и отражать общую переносимость побочных эффектов, а также симптоматику, связанную с заболеванием. За исключением болей, больные в группе паклитаксела давали немного лучшие оценки в отношении качества жизни по каждому из параметров во время лечения по сравнению с больными в группе CMFP. Хотя настоящее испытание представляет собой первое сравнение монотерапии паклитакселом и CMFP в качестве химиотерапии первой линии при метастатическом заболевании, предварительные результаты двух других испытаний помогают представить настоящие результаты в соответствующем контексте [47-49]. Sledge с соавт. [48] из ECOG провели сравнение паклитаксела в форме монотерапии, вводимого в дозе 175 мг/м2 на протяжении 24 часов, с доксорубицином и с комбинацией доксорубицин+паклитаксел в рандомизированном испытании, проведенном на ранее не леченых больных с метастатическим раком молочной железы. В этом испытании процент объективных ответов при монотерапии паклитакселом составлял 34%, а медиана времени до прогрессии составляла 6 месяцев. Эти результаты близки к описанным в нашем испытании. В испытании ECOG сравнение двух индивидуальных препаратов, паклитаксела и доксорубицина, привело к выводу об аналогичном ответе, времени до прогрессии и выживаемости. Однако в обеих группах монотерапии имел место перекрест. В исследовании Gianni с соавт. [50] у 41% больных с распространенным раком молочной железы, получавших лечение комбинацией паклитаксел/доксорубицин, был достигнут полный ответ, общий процент объективных ответов был равен 94%, а продолжительность частичного ответа составляла 11 месяцев. Последующие испытания по фазе II дали процент ответа ≥58% [47, 51, 52]. В ранее упоминавшемся рандомизированном испытании ECOG объективный процент ответа при использовании комбинации паклитаксел/доксорубицин составлял 47%, причем полные ответы наблюдались лишь у 6% больных, а медиана времени до прогрессии составляла 8 месяцев [48]. Выживаемость больных во всех трех группах испытания ECOG была одинаковой. В вызвавшем противоречивые отклики предварительном отчете о рандомизированном испытании по раку молочной железы Европейской организации по исследованию и лечению рака (EORTC) отмечался лучший ответ и лучшее время до прогрессии при использовании монотерапии доксорубицином по сравнению с монотерапией паклитакселом, однако выживаемость и качество жизни были одинаковыми, а токсичность была большей в группе доксорубицина. Исследования ECOG и EORTC позволяют полагать, что монотерапия паклитакселом или доксорубицином с последующим переводом больных на другой агент является эффективной в качестве исходной комбинированной терапии при лечении метастатического заболевания, однако процент ответа и исходное время до прогрессии являются худшими в случае монотерапии по сравнению с использованием комбинации. Настоящее испытание является единственным из всех трех, в которых не осуществлялось перевода больных на применение таксана, а в обеих группах больных в последующем переводили на лечение антрациклинами. Таким образом, настоящее испытание может указывать, что последовательность паклитаксел! антрациклин может давать преимущество по выживаемости по сравнению с последовательностью CMFP! антрациклин. Это важно для нахождения оптимальной последовательности применения в испытаниях по адъювантной терапии. Bonadonna с соавт. [53] сообщили о превосходстве схем с блочным применением доксорубицина и CMF при адъювантной терапии у больных с плохим прогнозом по сравнению с чередованием доксорубицина и CMF (по схеме курс одного с последующим курсом другого). Высказывалось предположение, что использование чередующейся химиотерапии агентами, не дающими перекрестной устойчивости, является оптимальным для индукции гибели клеток [54-57]. Наше испытание дает дополнительную информацию относительно оптимальной последовательности блоков химиотерапии и позволяет предположить, что паклитаксел с последующим применением антрациклина может быть лучшей схемой по сравнению с использованием CMFP и последующим применением антрациклина, хотя исходное подавление опухоли является одинаковым. Одна из гипотез заключается в том, что паклитаксел и доксорубицин дают меньшую перекрестную резистентность по отношению друг к другу по сравнению с CMFP. Таким образом, могут существовать оптимальные последовательности применения новых схем с использованием блочной химиотерапии, особенно при адъювантном лечении. В пользу этой гипотезы могут говорить предварительные результаты по применению адъювантной терапии в группе CALGB, когда была показана лучшая выживаемость при использовании блока курсов паклитаксела после исходного лечения схемой доксорубицин/циклофосфамид при раке молочной железы с поражением лимфоузлов [58]. Эти данные требуют дальнейших наблюдений и подтверждения. CMFP использовалась у сильно ослабленных больных с метастатическими формами рака молочной железы, где задача терапии заключается в достижении паллиативного результата. Настоящее испытание показывает, что паклитаксел дает меньшую миелосупрессию и меньшее число инфекций чем CMFP; кроме того, он дает меньше побочных эффектов, требующих госпитализации, обеспечивает эквивалентное исходное подавление опухоли и обеспечивает важное для клиники повышение выживаемости.
Литература
1. Boring CC, Squires TS, Long T: Cancer statistics. CA Cancer J Clin 42:19-38, 1992
2. Brambilla C, DeLena M, Rossi A, et al: Response and survival in advanced breast cancer after two non-cross-resistant combinations. BMJ 1:801-804, 1976
3. Canellos GP, Pocock SJ, Taylor SG, et al: Combination chemotherapy for metastatic breast carcinoma. Cancer 38:1882-1886, 1976
4. Tranum В, Hoogstraten В, Kennedy A, et al: Adriamycin in combination for the treatment of breast cancer. Cancer 41:2078-2083, 1978
5. Cooper RG: Combination chemotherapy in hormone resistant breast cancer. Proc Am Assoc Cancer Res 10:15, 1969 (abstr)
6. Muss HB, White DR, Cooper MR, et al: Combination chemotherapy in advanced breast cancer: A randomized trial comparing a three- vs a five-drug program. Arch Intern Med 137:1711-1714, 1977
7. Smalley RV, Murphy S, Huguley CM, et al: Combination versus sequential five-drug chemotherapy in metastatic carcinoma of the breast. CancerRes36:3911-3916, 1976
8. Hoogstraten В, George SL, Samal B, et al: Combination chemotherapy and Adriamycin in patients with advanced breast cancer: A Southwest Oncology Group study. Cancer 38:13-20, 1976
9. Cebon JS, Bishop JF, Harvey V, et al: Dose-intense weekly cyclophosphamide, methotrexate, 5-fluorouracil, vincristine and prednisolone (CMFP) in advanced breast cancer. Br J Cancer 61:133-136, 1990
10. Canellos GP, DeVita VT, Gold GL, et al: Combination chemotherapy for advanced breast cancer: Response and effect on survival. Ann Intern Med 84:389-392, 1976
11. Cummings FJ, Gelman R, Horton J: Comparison ofCAF versus CMFP in metastatic breast cancer: Analysis of prognostic factors. J Clin Oncol 3:932-940, 1985
12. Coates A. Gebski V. Bishop JF.etal: Improving the quality of life during chemotherapy for advanced breast cancer: A comparison of intermittent and continuous treatment strategies. N Engl J Med 317:1490-1495,1987
13. Aisner J, Weinberg V, Perloff M, et al: Chemotherapy vs chemoimmunotherapy (CAP v CAFVP v CMF each ± MER) for metastatic carcinoma of the breast: A CALGB study. J Clin Oncol 5:1523-1533, 1987
14. Creech RH, Catalano RB, Mastrangelo MJ, et al: An effective low-dose intermittent cyclophosphamide, methotrexate. and 5-fluorouracil treatment regimen for metastatic breast cancer. Cancer 35:1101-1107,1975
15. Carbone PP, Bauer M, Band P, et al: Chemotherapy of disseminated breast cancer. Cancer 39:2916-2922, 1977
16. Tormey DC, Gelman R, Band PR, et al: Comparison of induction chemotherapies for metastatic breast cancer. Cancer 50:1235-1244, 1982
17. Henderson IC: Principles in the management of metastatic disease, in Harris JR, Hellman S, Henderson IC, et al (eds): Breast Diseases. Philadelphia, PA, Lippincott, 1992, pp 604-673
18. Bull JM. Tormey DC, Li S-H, et al: A randomized comparative trial of Adriamycin versus methotrexate in combination drug therapy. Cancer 41:1649-1657,1978
19. Muss HB, White DR, Richards F II, et al: Adriamycin versus methotrexate in five-drug combination chemotherapy for advanced breast cancer. Cancer 42:2141-2148, 1978
20. Bezwoda WR, de Moor NO, Derman D, et al: Combination chemotherapy of metastatic breast cancer. Cancer 44:392-397, 1979
21. Tormey DC, Weinberg VE, Holland JF, et al: A randomized trial of five and three drug chemotherapy and chemoimmunotherapy in women with operable node positive breast cancer. J Clin Oncol 1:138-145,1983
22. Brincker H, Rose С, von der Maase H, et al: A randomized study of CAF + ТАМ (tamoxifen) versus CMF + ТАМ in metastatic breast cancer. Proc Am Soc Clin Oncol 3:113, 1984 (abstr)
23. Tormey DC, Weinberg VE, Leone LA, et al: A comparison of intermittent vs continuous and of Adriamycin vs methotrexate 5-drug chemotherapy for advanced breast cancer. Am J Clin Oncol 7:231-239, 1984
24. Fossati R, Confalonieri C. Tom V, et al: Cytotoxic and hormonal treatment for metastatic breast cancer: A systematic review of published randomized trials involving 31,510 women. J Clin Oncol 16:3439-3460,1998
25. Horwitz SB: Mechanism of action of Taxol. Trends Pharmacol Sci 13:134-136, 1992
26. Rao S, Horwitz SB, Ringel 1: Direct photoaffinity labelling of tubulin with Taxol. J Natl Cancer Inst 84:785-788. 1992
27. Rowinsky EK, Burke PJ, Karp JE, et al: Phase 1 and pharmacodynamic study of Taxol in refractory acute leukemias. Cancer Res 49:4640-4647, 1989
28. Holmes FA, Walters RS, Theriault RL, et al: Phase II trial of Taxol. an active drug in the treatment of metastatic breast cancer. J Natl Cancer Inst 83:1797-1805, 1991
29. O'Shaughnessy JA, Cowan KH: Current status of paclitaxel in the treatment of breast cancer. Breast Cancer Res Treat 33:27-37, 1995
30. Seidman AD, Reichman BS. Crown JPA. et al: Paclitaxel as second and subsequent therapy for metastatic breast cancer: Activity independent of prior anthracycline response. J Clin Oncol 13:1152-1159,1995
31. Gianni L, Capri G. Munzone E, et al: Paclitaxel (Taxol) efficacy in patients with advanced breast cancer resistant to anthracyclines. Semin Oncol 21:29-33. 1994 (Suppl 8)
32. Wilson WH, Berg SL, Bryant G. et al: Paclitaxel in doxorubicin-refractory or mitoxantrone-refractory breast cancer: A phase 1/11 trial of 96-hour infusion. J Clin Oncol 12:1621-1629, 1994
33. Gelmon К, Nabholtz JM, Bontenbal M: Randomized trial of two doses of paclitaxel in metastatic breast cancer after failure of standard therapy. Ann Oncol 5:198-201, 1994 (Suppl 5)
34. Eisenhauer EA, ten Bokkel Huinink WW, Swenerton KD, et al: European-Canadian randomized trial of paclitaxel in relapsed ovarian cancer: High-dose versus low-dose and long versus short infusion. J Clin Oncol 12:2654-2666, 1994
35. Schiller JH, Storer B, Tutsch K. et al: A phase I trial of 3-hour usions of paclitaxel (Taxol) with or without granulocyte colony stimulating factor. Semin Oncol 21:9-14, 1994(,suppl 8)
36. Greco FA, Hainsworth JD: One-hour paclitaxel infusion schedule: A phase I/II comparative trial. Semin Oncol 22:118-123. 1995 (Suppl. 6)
37. Michael M, Bishop JF. Levi JA, et al: Australian multicentre Phase II trial of paclitaxel in women with metastatic breast cancer and or chemotherapy. Med J Aust 166:520-523. 1997
38. Rischin D, Webster LK, Millward MJ, et al: Cremophor pharmacokinetics in patients receiving 3-, 6- and 24-hour infusions of rlitaxel. J Natl Cancer Inst 88:1297-1301, 1996
39. Holmes FA, Valero V, BuzdarAU, et al: Final results: Randomized phase III trial of paclitaxel by 3 hour versus 96 hour infusion in patients (pt) with met breast cancer (MBC). Proc Am Soc Clin Oncol:110a. 1998 (abstr 426)
40. Holmes FA, Walters RS, Theriault RL, et al: Phase II trial of Taxol, an active drug in the treatment of metastatic breast cancer. J Natl Cancer Inst 83:1797-1805,!991
41. Reichman BS, Seidman AD, Crown JPA, et al: Paclitaxel and recombinant human granulocyte colony-stimulating factor as initial chemotherapy for metastatic breast cancer. J Clin Oncol 11:1943-1951, 93
42. Nabholtz JM. Gelmon К. Bontenbal M, et al: Multicenter. randomized comparative study of two doses of paclitaxel in patients th metastatic breast cancer. J Clin Oncol 14:1858-1867, 1996
43. Miller AB, Hoogstraten B, Staquet M, etal: Reporting results of cancer treament. Cancer 47:207-214. 1981
44. Therapeutic Goods Administration: Guidelines for Good Clinical Research Practice (GCRP) in Australia. Canberra. Australia, Commonwealth Government Publisher, 1981
45. Spitzer WO, Dobson AJ, Hall J, et al: Measuring the quality of life of cancer patients: A concise QL-index for use by physicians. J Chron Dis 34:585-597, 1981
46. Matthews JNS. Altman DG, Campbell MJ, et al: Analysis of serial measurements in medical research. BMJ 300:230-235. 1990
47. Sledge GW. Robert N. Sparano JA. et al: Eastern Cooperative Oncology Group studies of paclitaxel and doxorubicin in advanced breast cancer. Semin Oncol 22:105-108, 1995 (Suppl 6)
48. Sledge GW, Neuberg D. Ingle J, et al: Phase III trial of doxorubicin vs. paclitaxel vs. doxorubicin + paclitaxel as first-line therapy for metastatic breast cancer: An intergroup trial. Proc Am Soc Clin Oncol 16:la. 1997 (abstr)
49. Gamucci T. Piccart M, Bruning P, et al: An EORTC crossover trial comparing singleagent Taxol and doxorubicin as first- and second-line chemotherapy in advanced breast cancer. Proc Am Soc Clin Oncol 16:154a, 1997 (abstr)
50. Gianni L, Munzone E. Capri G, et al: Paclitaxel by 3-hour infusion in combination with bolus doxorubicin in women with untreated metastatic breast cancer: High antitumor efficacy and cardiac effects in a dose-finding and sequence-finding study. J Clin Oncol 13:2688-2699, 1995
51. Hortobagyi GN, Holmes FA: Optimal dosing of paclitaxel and doxorubicin in metastatic breast cancer. Semin Oncol 24:4-7, 1997 (Suppl 3)
52. Gehl J, Boesgaard M, Paaske T, et al: Paclitaxel and doxorubicin in metastatic breast cancer. Semin Oncol 23:35-38, 1996 (Suppl 15)
53. Bonadonna G. Zambetti M, Valagussa P: Sequential or alternating doxorubicin and CMF regimens in breast cancer with more than 3 positive nodes: Ten year results. JAMA 273:542- 547, 1995
54. Norton L, Simon R: The Norton-Simon hypothesis revisited. Cancer Treat Rep 70:163- 169. 1986
55. Norton L: A Gompertzian model of human breast cancer growth. Cancer Res 48:7067- 7071. 1988
56. Surbone A, Norton L: Kinetic concepts in the treatment of breast cancer. Ann NYAcad Sci 698:48-62, 1993
57. Hudis С. Seidman A, Raptis G, et al: Sequential adjuvant therapy: The Memorial Sloan- Kettering Cancer Center Experience. Semin Oncol 23:58-64. 1996 (Suppl 1)
58. Henderson IC. Berry D. Demetri G. et al: Improved disease-free (DFS) and overall survival (OS) from the addition of sequential paclitaxel (T) but not from the escalation of doxorubicin (A) dose level in the adjuvant chemotherapy of patients (pts) with node-positive primary breast cancer (ВС). Proc Am Soc Clin Oncol 17:101a, 1998 (abstr 390A)
Рис. 1. Схема испытания
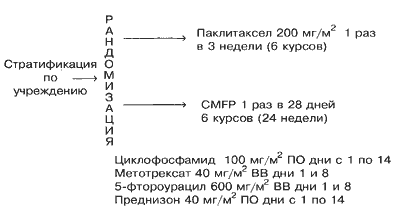
Рис. 2. Выживаемость без прогрессии в зависимости от группы лечения
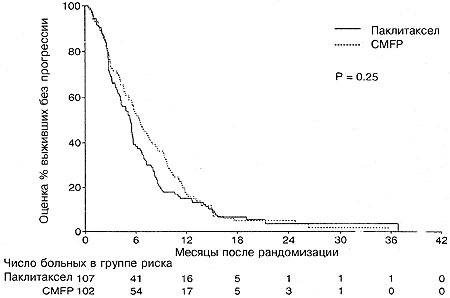
Рис. 3. Общая выживаемость в зависимости от группы лечения
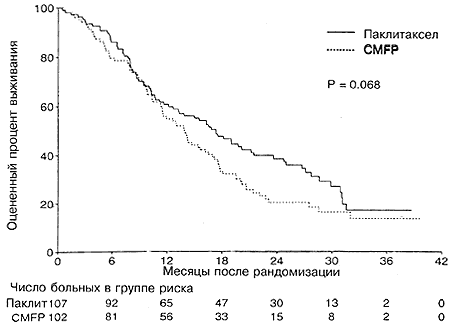
Рис. 4. Среднее изменение качества жизни по отношению к исходному в зависимости от группы лечения