ќрганические соединени€, молекулы которых содержат карбонильную группу  , называютс€ карбонильными соединени€ми. ¬ зависимости от характера св€занных с карбонильной группой заместителей карбонильные соединени€ дел€тс€ на альдегиды, кетоны, карбоновые кислоты и их функциональные производные.
, называютс€ карбонильными соединени€ми. ¬ зависимости от характера св€занных с карбонильной группой заместителей карбонильные соединени€ дел€тс€ на альдегиды, кетоны, карбоновые кислоты и их функциональные производные.
јЋ№ƒ≈√»ƒџ
јльдегидами называютс€ органические соединени€, содержащие карбонильную группу, в которой атом углерода св€зан с радикалом и одним атомом водорода, то есть обща€ формула альдегидов 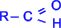 . »сключение составл€ет муравьиный альдегид
. »сключение составл€ет муравьиный альдегид  , в котором, как видно, R=H.
, в котором, как видно, R=H.
»зомери€
ƒл€ альдегидов характерна изомери€ углеводородного радикала, который может иметь как нормальную (неразветвленную) цепь, так и разветвленную, а также межклассова€ изомери€ с кетонами. Ќапример,
| O II CH3ЦCH2ЦCH2ЦC I H | O II CH3ЦCHЦC I I H CH3 | O II CH3ЦCH2ЦC | ЦCH3 |
| масл€ный альдегид или бутаналь | изомасл€ный альдегид или 2-метил-пропаналь | метилэтилкетон или бутанон -2 |
ѕолучение
1. Ќаиболее часто примен€ющимис€ способами получени€ альдегидов €вл€ютс€ окисление и каталитическое дегидрирование первичных спиртов.
a) ќкисление первичных спиртов.
ак видно, при дальнейшем окислении образуютс€ кислоты. Ёти реакции приводились уже при рассмотрении химических свойств спиртов.
b) ƒегидрирование первичных спиртов. –еакцию провод€т, пропуска€ пары спирта над нагретым до 200-300∞— катализатором, в качестве которого используютс€ медь, никель, кобальт и др.
| O II RЦCH2ЦOH ЦЦt∞,katЃ RЦC I H | (альдегид) + H2 |
2. –азработан метод получени€ уксусного альдегида окислением этилена кислородом воздуха в присутствии солей меди и паллади€.
| O II 2CH2=CH2 + O2 ЦЦCuCl2,PbCl2Ѓ 2CH3ЦC I H уксусный альдегид |
3. ”ксусный альдегид получают гидратацией ацетилена по реакции учерова.
| O II | ||
| HCºCH + H2O ЦЦHgSO4Ѓ [H2 | C=C | H] ЦЦЃ CH3ЦC |
 вини- ловый спирт вини- ловый спирт
| I H уксусный альдегид |
ѕодробно реакци€ учерова уже рассматривалась при изучении химических свойств ацетиленовых углеводородов.
4. јльдегиды получают гидролизом дигалогенопроизводных углеводородов, однако только тех, у которых оба атома галогена расположены у одного из концевых атомов углерода.
| CH3ЦCH2Ц | 
| + 2H2O Ѓ [CH3ЦCH2Ц | 
| ] + 2HCl |
| 1,1- дихлорпропан | 1,1-пропандиол | | ¯ |
| O II CH3ЦCH2ЦC I H | + H2O |
| пропаналь |
ѕри действии воды на дигалогеналкил в щелочной или кислой среде реакци€ его гидролиза проходит стадию образовани€ двухатомного спирта, содержащего две гидроксильных группы у одного атома углерода.
“акие спирты вследствие своей неустойчивости в момент образовани€ тер€ют воду и образуют альдегиды.
‘изические свойства
ѕростейший альдегид Ц муравьиный Ц газ с весьма резким запахом. ƒругие низшие альдегиды Ц жидкости, хорошо растворимые в воде. јльдегиды обладают удушливым запахом, который при многократном разведении становитс€ при€тным, напомина€ запах плодов. јльдегиды кип€т при более низкой температуре, чем спирты с тем же числом углеродных атомов. Ёто cв€зано с отсутствием в альдегидах водородных св€зей. ¬ то же врем€ температура кипени€ альдегидов выше, чем у соответствующих по молекул€рной массе углеводородов, что св€зано с высокой пол€рностью альдегидов.
‘изические свойства некоторых альдегидов представлены в таблице.
|
|
|
“аблица. ‘изические свойства некоторых альдегидов
| Ќазвание | ‘ормула | t∞кип., ∞C | t∞пл., ∞C | d420 |
| ћуравьиный альдегид | O II HЦC I H | -92,0 | -21,0 | 0,815 (при 20∞—) |
| ”ксусный альдегид | O II CH3ЦC I H | -123,5 | 21,0 | 0,780 |
| ѕропионовый альдегид | O II CH3ЦCH2ЦC I H | -102,0 | 48,8 | 0,807 |
| ћасл€ный альдегид | O II CH3ЦCH2ЦCH2ЦC I H | -99,0 | 75,7 | 0,817 |
| »зомасл€ный альдегид | O II CH3ЦCHЦC I I CH3 H | -65,9 | 64,0 | 0,794 |
’имические свойства
јльдегиды характеризуютс€ высокой реакционной способностью. Ѕольша€ часть их реакций обусловлена наличием карбонильной группы. јтом углерода в карбонильной группе находитс€ в состо€нии sp2 -гибридизации и образует три s- св€зи (одна из них Ц св€зь —Цќ), которые расположены в одной плоскости под углом 120∞ друг к другу.

—хема строени€ карбонильной группы
ƒвойна€ св€зь карбонильной группы по физической природе сходна с двойной св€зью между углеродными атомами, т. е. это сочетание s- и p- св€зей, последн€€ из которых образована р- электронами атомов углерода и кислорода. ¬виду большей электроотрицательности атома кислорода по сравнению с атомом углерода, св€зь —=ќ сильно пол€ризована за счет смещени€ электронной плотности p- св€зи к атому кислорода, в результате чего на атоме кислорода возникает частичный отрицательный (d-), а на атоме углерода Ц частичный положительный (d+) зар€ды: 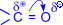 .
.
Ѕлагодар€ пол€ризации атом углерода карбонильной группы обладает электрофильными свойствами и способен реагировать с нуклеофильными реагентами. ¬ажнейшими реакци€ми альдегидов €вл€ютс€ реакции нуклеофильного присоединени€ по двойной св€зи карбонильной группы.
1. ќдной из типичных реакций нуклеофильного присоединени€ альдегидов €вл€етс€ присоединение синильной (циановодородной) кислоты, привод€щее к образованию a- оксинитрилов.
| CH3Ц | 
| + 
| OH I ЦЦKCNЃ CH3ЦCЦCN I H | |
Ёта реакци€ используетс€ дл€ удлинени€ углеродной цепи и получени€ a- оксикислот.
2. ѕрисоединение гидросульфита натри€ дает кристаллические вещества, обычно называемые гидросульфитными производными альдегидов.
| O II CH3ЦC I H | + HSO3Na Ѓ CH3Ц | OH I CЦSO3Na (гидросульфитное производное уксусного альдегида) I H |
”пом€нутые производные легко гидролизуютс€ в любых средах, привод€ к исходному карбонильному соединению. “ак, при нагревании с раствором соды гидросульфитного производного уксусного альдегида образуетс€ собственно уксусный альдегид.
| 2CH3Ц | OH I CЦSO3Na + Na2CO3 Ѓ 2CH3Ц I H | O II C + 2Na2SO3 + CO2 + H2O I H |
ƒанное свойство используетс€ дл€ очистки альдегидов и выделени€ их из смесей.
3. ѕрисоединение спиртов к альдегидам приводит к образованию полуацеталей Ц соединений, в которых атом углерода св€зан и с гидроксильной (ЦќЌ), и с алкоксильной (ЦќR) группами.
|
|
|
| RЦ | 
| OH I ЂRЦCH I OC2H5 (полуацеталь) |
ѕри обработке полуацеталей избытком спирта в кислой среде образуютс€ ацетали Ц соединени€, в которых атом углерода св€зан с двум€ алкоксильными группами (реакци€ напоминает синтез простых эфиров из спиртов).
| RЦ |  полуацеталь полуацеталь
| + HЦOC2H5 | H+Ѓ | RЦ |  ацеталь ацеталь
| + H2O |
¬ отличие от простых эфиров ацетали гидролизуютс€ под действием кислот с образованием спирта и альдегида.
4. ѕрисоединение водорода к альдегидам осуществл€етс€ в присутствии катализаторов (Ni, Co, Pd и др.) и приводит к образованию первичных спиртов.
| O II RЦC + H2 ЦЦNiЃ RЦCH2ЦOH(первичный спирт) I H |
¬се чаще в качестве восстанавливающего агента примен€ют алюмогидрид лити€ LiAlH4 и борогидрид натри€ NaBH4.
ѕомимо реакций присоединени€ по карбонильной группе дл€ альдегидов характерны также реакции окислени€.
5. ќкисление. јльдегиды легко окисл€ютс€, образу€ соответствующие карбоновые кислоты.
| O II RЦC I H | ЦЦ[O]Ѓ | O II RЦC (кислота) I OH |
a) аммиачный раствор оксида серебра [Ag(NH3)2]OH при нагревании с альдегидами окисл€ет альдегид до кислоты (в виде ее аммониевой соли) с образованием свободного металлического серебра. ¬осстановленное серебро ложитс€ тонким слоем на стенки химического сосуда, в котором осуществл€етс€ реакци€, и получаетс€ серебр€ное зеркало. Ёта реакци€, получивша€ поэтому название "серебр€ного зеркала", служит качественной реакцией на альдегиды.
| O II CH3ЦC+ 2[Ag(NH3)2OH ЦЦt∞Ѓ CH3COONH4(ацетат аммони€) + 2Ag¯ + 3NH3 + H2O I H |
b) еще одной характерной реакцией €вл€етс€ окисление альдегидов гидроксидом меди (II).
| O II CH3ЦC I H | + 2Cu(OH)2(голубой) ЦЦt∞Ѓ CH3Ц | O II C (уксусна€ к-та) + Cu2O¯(красный) +2H2O I OH |
ѕри нагревании голубого гидроксида меди (II) с раствором уксусного альдегида выпадает красный осадок оксида меди (I). ѕри этом уксусный альдегид окисл€етс€ до уксусной кислоты, а медь со степенью окислени€ +2 восстанавливаетс€ до меди со степенью окислени€ +1. ћуравьиный альдегид (формальдегид) занимает особое место в р€ду альдегидов. ¬ св€зи с отсутствием у муравьиного альдегида радикала, ему присущи некоторые специфические свойства. ќкисление формальдегида, например, осуществл€етс€ до двуокиси углерода —ќ2.
‘ормальдегид легко полимеризуетс€ с образованием циклических и линейных полимеров. “ак, в кислой среде он образует циклический тример Ц триоксиметилен.

| =O | H+ ЦЃ |  (триоксиметилен) (триоксиметилен)
|
—ухой газообразный формальдегид в присутствии катализаторов образует высокомолекул€рный полиформальдегид. ѕолимеризаци€ формальдегида напоминает полимеризацию алкенов.

| =O + | 
| =O ЦЦkatЃ | H I ЕЦC I H | H I ЦOЦCЦOЦЕ I H |  ЦЦЦЦЦЃ ЦЦЦЦЦЃ
| ЕЦH2CЦO |
¬ водных растворах формальдегид образует полимер, называемый параформом.
n CH2 = O + H2O Ѓ HOCH2(OCH2)n-2OCH2OH
(параформ)
ќсобое практическое значение имеет реакци€ поликонденсации формальдегида с фенолом с образованием фенолформальдегидных смол. ѕри действии щелочных или кислых катализаторов на смесь фенола и формальдегида конденсаци€ идет в орто- и пара- положени€х.

| H+ или OH- | 
|
| ЦЦЦЦЦЦЦЦЦЦЦЦЦЃ |
–ост молекулы за счет конденсации фенола с формальдегидом осуществл€етс€ при нормальной температуре в линейном направлении.

| 
| Ѓ H2O + | 
| 
|

|  
| Ѓ | 
| 
| CH2OH / |
и т. д.
—уммарно реакцию поликонденсации фенола с формальдегидом можно изобразить следующим образом:
| n | 
| = O + (n+1) | 
| катализатор | 
| 
| + nH2O |
| ЦЦЦЦЦЦЦЦЃ |
‘енолформальдегидные смолы Ц первенцы промышленных синтетических смол, их производство под названием "бакелит" впервые начато в 1909 году. ‘енолформальдегидные смолы используютс€ в производстве различных пластмасс. ¬ сочетании с различными наполнител€ми такие пластмассы называютс€ фенопластами. роме того, фенолформальдегидные смолы примен€ютс€ при изготовлении различных клеев и лаков, термоизол€ционных материалов, древесных пластиков, литейных форм и др.
|
|
|
ѕрименение
”же много упом€нуто о применении формальдегида. роме того, он используетс€ дл€ получени€ карбамидных смол при взаимодействии с мочевиной, на основе которых производ€тс€ пластмассы, необходимые дл€ нужд электротехники. –астворы формальдегида (формалин) используютс€ в кожевенной промышленности дл€ дублени€ кож, дл€ дезинфекции зерно- и овощехранилищ, теплиц, парников, дл€ протравливани€ сем€н перед посевом, дл€ хранени€ анатомических препаратов, а также в производстве некоторых лекарственных препаратов.
”ксусный альдегид €вл€етс€ исходным сырьем дл€ получени€ в промышленном масштабе уксусной кислоты, уксусного ангидрида, этилового спирта, этилацетата и других ценных продуктов, а при конденсации с аминами и фенолами Ц различных синтетических смол.
≈“ќЌџ
етонами называютс€ соединени€, в молекуле которых карбонильна€ группа св€зана с двум€ углеводородными радикалами. ќбща€ формула кетонов  , где R может совпадать с R'.
, где R может совпадать с R'.
»зомери€
ƒл€ кетонов характерна изомери€ углеводородных радикалов, изомери€ положени€ карбонильной группы и межклассова€ изомери€ с альдегидами.
ѕолучение
ѕочти все способы получени€, приведенные ранее дл€ альдегидов (см. "јльдегиды. ѕолучение"), применимы и дл€ кетонов.
1. ќкисление вторичных спиртов.
| R \ CHЦOH(вторичный спирт) ЦЦ [O]Ѓ / RТ | R \ C=O(кетон) + H2O / RТ |
2. ƒегидрирование вторичных спиртов.
| R \ CHЦOH(вторичный спирт) ЦЦkatЃ / RТ | R \ C=O(кетон) + H2 / RТ |
3. √идратаци€ гомологов ацетилена (реакци€ учерова).
| RЦCºCH + HЦOH ЦHgSO4Ѓ [RЦ | CЦCH2] Ѓ RЦ I ø OH | CЦCH3 II O |
4. √идролиз дигалогенопроизводных углеводородов, содержащих оба атома галогена у одного из средних в цепи углеродных атомов.
| CH3Ц | Cl I CЦCH3(2,2- дихлорпропан) + 2H2O Ѓ [CH3Ц I Cl | 
| CH3](2,2- пропандиол) + 2HCl |
| ¯ | |||
| CH3Ц | O II CЦCH3 + H2O(диметилкетон (ацетон)) |
5. етоны получают, кроме того, пиролизом кальциевых солей карбоновых кислот при их нагревании.
| O II CH3ЦC I O | O | |
| \ Ca(уксуснокислый кальций) Ц t∞Ѓ CH3Ц / | II CЦCH3 + CaCO3 | |
| O II CH3ЦC I O |
‘изические свойства
Ќизшие кетоны Ц жидкости, легко растворимые в воде. ¬ основном, кетоны обладают при€тным запахом, напоминающим запах цветов. ак и альдегиды, кетоны кип€т при более низкой температуре, чем соответствующие спирты, однако выше, чем углеводороды. ‘изические свойства некоторых кетонов представлены в таблице.
“аблица. ‘изические свойства некоторых кетонов
| Ќазвание | ‘ормула | t∞пл., ∞C | t∞кип., ∞C | d420 |
| јцетон (диметилкетон) | CH3Ц  ЦCH3 ЦCH3
| -95,35 | 56,2 | 0,790 |
| ћетилэтилкетон | CH3Ц  ЦCH2ЦCH3 ЦCH2ЦCH3
| -86,4 | 79,6 | 0,805 |
| ћетилпропилкетон | CH3Ц  ЦCH2ЦCH2ЦCH3 ЦCH2ЦCH2ЦCH3
| -77,8 | 101,7 | 0,809 |
| ƒиэтилкетон | CH3ЦCH2Ц  ЦCH2ЦCH3 ЦCH2ЦCH3
| -42,0 | 102,7 | 0,816 |
’имические свойства
ак и альдегиды, кетоны характеризуютс€ высокой реакционной способностью. ’имическа€ активность альдегидов и кетонов тем выше, чем больше положительный зар€д на атоме углерода карбонильной группы. –адикалы, увеличивающие этот положительный зар€д, резко повышают реакционную способность альдегидов и кетонов, а радикалы, уменьшающие поло-жительный зар€д, оказывают противоположное действие. ¬ кетонах две алкильные группы €вл€ютс€ электронодонорными, откуда становитс€ пон€тным, почему кетоны менее активны в реакци€х нуклеофильного присоединени€ по сравнению с альдегидами.
ѕримеры реакций этого типа дл€ альдегидов подробно рассмотрены ранее (см. "јльдегиды. ’имические свойства"), поэтому, привод€ некоторые примеры реакций нуклеофильного присоединени€ по карбонильной группе кетонов, уделим внимание лишь отличи€м их химических свойств от альдегидов.
|
|
|
1. ѕрисоединение синильной кислоты.
| R \ C=O(кетон) + HЦ CN ЦKCNЃ CH3Ц / RТ | OH I CЦCN(нитрил a-оксиизомасл€ной кислоты) I CH3 |
2. ѕрисоединение гидросульфита натри€.
| R \ C=O(кетон) + HSO3Na Ѓ RЦ / RТ | OH I CЦSO3Na(гидросульфитное производное кетона) I RТ |
—ледует отметить, что в реакцию с гидросульфитом натри€ вступают только метилкетоны, т. е. кетоны, имеющие группировку CH3.
3. ѕо сравнению с альдегидами дл€ кетонов не характерны реакции со спиртами.
4. ѕрисоединение водорода. ѕрисоединение водорода к кетонам приводит к образованию вторичных спиртов.
| R \ C=O(кетон) + H2 ЦNiЃ / RТ | R \ CHЦOH(вторичный спирт) / RТ |
5. етоны окисл€ютс€ значительно труднее, чем альдегиды. ислород воздуха и слабые окислители не окисл€ют кетоны. етоны не дают реакции "серебр€ного зеркала" и не реагируют с гидроксидом меди (II). ѕри действии сильных окислителей в жестких услови€х углеродна€ цепь молекулы кетона разрушаетс€ р€дом с карбонильной группой и образуютс€ кислоты (иногда кетоны в зависимости от строени€ исходного кетона) с меньшим числом атомов углерода.

| 
| 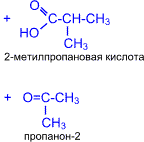
|
ѕрименение
Ќаиболее широкое промышленное применение имеет простейший представитель кетонов Ц ацетон. јцетон €вл€етс€ ценным растворителем, использующимс€ в лакокрасочной промышленности, в производстве искусственного шелка, кинопленки,бездымного пороха. ќн служит также исходным сырьем при производстве метакриловой кислоты, метилметакрилата (производство небьющегос€ органического стекла), метилизобутилкетона и др.






