¬ основ≥ комплексонометричного титруванн€ лежить реакц≥€ утворенн€ комплексних сполук м≥ж анал≥зованою речовиною та орган≥чними реагентами комплексонами. омплексони представл€ють собою пох≥дн≥ пол≥ам≥нокарбонових кислот.
омплексон ≤≤≤ (трилон Ѕ) представл€Ї собою слабку чотирьохосновну етилендиам≥нтетраацетатну кислоту або њњ динатр≥Їву с≥ль − Na2H2Y:

 NaOOC Ц CH2 CH2 Ц COOH
NaOOC Ц CH2 CH2 Ц COOH

 N − CH2 −CH2 − N
N − CH2 −CH2 − N
HOOC Ц CH2 CH2 Ц COONa.
омплексон ≤≤≤ утворюЇ м≥цн≥ комплекси майже з ус≥ма металами, за вин€тком лужних, добре розчин€Їтьс€ в вод≥ (108 г/дм3 при температур≥ 22 ∞—), при титруванн≥ легко встановити точку екв≥валентност≥.
—т≥йк≥сть комплекс≥в кат≥он≥в метал≥в з трилоном Ѕ залежить в≥д природи металу та рЌ середовища. –егулюючи кислотн≥сть середовища, можна провести диференц≥йоване визначенн€ кат≥он≥в в сум≥шах.
ƒл€ приготуванн€ робочого розчину в комплексонометр≥њ користуютьс€ х≥м≥чно чистим диг≥дратом Na2H2YЈ2H2O.
≤ндикатори в комплексонометр≥њ. ƒл€ ф≥ксуванн€ к≥нцевоњ точки титруванн€ в комплексонометр≥њ використовують метало≥ндикатори. « х≥м≥чноњ точки зору метало≥ндикатори Ц це орган≥чн≥ сполуки, що утворюють з ≥онами металу забарвлен≥ комплекси. ¬ точц≥ екв≥валентност≥ спостер≥гаЇтьс€ р≥зка зм≥на забарвленн€ розчину. ¬ переважн≥й б≥льшост≥ випадк≥в користуютьс€ такими ≥ндикаторами: ер≥охром чорний “, мурексид, ксиленоловий оранжевий, кислотний хром темно-син≥й. ќрган≥чн≥ барвники ер≥охром чорний “ (≥нша назва хромоген чорний), а також кислотний хром темно-син≥й в лужному середовищ≥ набувають синього забарвленн€. ѕ≥д д≥Їю кат≥он≥в кальц≥ю, магн≥ю, цинку, алюм≥н≥ю ц≥ ≥ндикатори в област≥ рЌ 7 − 11 утворюють внутр≥шньокомплексн≥ сполуки винно-червоного кольору. ѕри титруванн≥ трилоном Ѕ кольоровий комплекс кат≥он металу Ц ≥ндикатор руйнуЇтьс€ ≥ утворюЇтьс€ б≥льш м≥цний безбарвний комплекс кат≥он металу Ц трилон Ѕ, ≥ндикатор зв≥льнюЇтьс€ ≥ в точц≥ екв≥валентност≥ розчин забарвлюЇтьс€ в син≥й кол≥р.
“итруванн€ розчину сполук Ca2+ та Mg2+трилоном Ѕ виконують т≥льки в лужному середовищ≥. ≤они г≥дрогену, що вид≥л€ютьс€ в результат≥ реакц≥њ взаЇмод≥њ кат≥он≥в досл≥джуваних метал≥в та ≥ндикатору, п≥дкислюють середовище, тому дл€ п≥дтримки сталост≥ pH в анал≥зований розчин додають амон≥ачну буферну сум≥ш (рЌ 9−11). ¬изначенню сполук кальц≥ю та магн≥ю заважають кат≥они важких метал≥в: Cu(≤≤), Fe (≤≤), Mn (≤≤).
2. —пособи виразу вм≥сту речовин в розчинах
1. ћол€рна концентрац≥€ екв≥валент≥в Ц в≥дношенн€ к≥лькост≥ екв≥валент≥в νекв(реч) розчиненоњ речовини до об'Їму розчину V, розм≥рн≥сть − моль екв/дм3:
c н(реч)=  .
.
÷€ концентрац≥€ найчаст≥ше використовуЇтьс€ в титриметричному анал≥з≥. «ручн≥сть використанн€ розчин≥в з мол€рною концентрац≥Їю екв≥валент≥в пол€гаЇ в тому, що саме ц≥ концентрац≥њ використовуютьс€ в закон≥ екв≥валентност≥, на €кому базуЇтьс€ титриметричний анал≥з: речовини реагують м≥ж собою в екв≥валентних сп≥вв≥дношенн€х. якщо позначити через ’ речовину, концентрац≥€ €коњ в розчин≥ визначаЇтьс€, а Y − титрант, то c н(’)∙ V (X) = c н(Y)Ј V (Y).
|
|
|
2. “итр розчиненоњ речовини − “ (’) − маса речовини в 1 см3 розчину:
“ (’) =  =
=  г/см3,
г/см3,
3. –озрахунки в титриметричному анал≥з≥
ќбчисленн€ в обТЇмному анал≥з≥ необх≥дн≥ €к дл€ встановленн€ концентрац≥њ титрованих розчин≥в, так ≥ дл€ визначенн€ результат≥в анал≥зу. –озрахунки в титриметр≥њ ірунтуютьс€ на закон≥ екв≥валентност≥. Ќезалежно в≥д методу титриметричного анал≥зу в точц≥ екв≥валентност≥ к≥льк≥сть екв≥валент≥в титранта дор≥внюЇ к≥лькост≥ екв≥валент≥в анал≥зованоњ речовини.
–озрахунки концентрац≥њ титрованих розчин≥в
ѕриклад 1. ƒл€ приготуванн€ стандартного розчину оксалатноњ кислоти наважку H2C2O4Ј2H2O масою 5,1752 г розчинили в м≥рн≥й колб≥ обТЇмом 250 см3. ќбчислити титр ≥ мол€рну концентрац≥ю речовини екв≥валента H2C2O4Ј2H2O (f екв(H2C2O4Ј2H2O) = 1/2).
–озв ʼ €занн€:
1. “ (H2C2O4Ј2H2O) −?
T (H2C2O4Ј2H2O) = 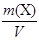 =
= 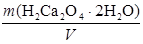 =
=  = 0,0207 г/см3.
= 0,0207 г/см3.
2) c н(H2C2O4Ј2H2O) −?
c н (H2C2O4Ј2H2O) =  =
=  =0,3284 ћ.
=0,3284 ћ.
M≈ (H2C2O4Ј2H2O) = f екв(H2C2O4Ј2H2O) Ј M (H2C2O4Ј2H2O) =1/2 Ј126,06 =
63,03 г/моль.
¬≥дпов≥дь: титр розчину складаЇ 0,02070 г/cм3; c (1/2 H2C2O4Ј2H2O) дор≥внюЇ 0,3284 ћ.
ƒл€ приготуванн€ стандартизованих розчин≥в спочатку готують розчин приблизноњ концентрац≥њ, €кий дал≥ титрують розчинами, концентрац≥€ €ких точно встановлена. ƒл€ цього обчислюють наважку речовини ’ з урахуванн€м мол€рноњ маси екв≥валента речовини M≈ (’), обТЇму розчину V (’) та заданоњ мол€рноњ концентрац≥њ екв≥валента речовини c н (’) в розчин≥:
m (X) = M≈ (’) Ј c н (’)Ј10−3,
де 10−3 − коеф≥ц≥Їнт перерахунку см3 в 1 дм3.
–озрахунок точноњ концентрац≥њ стандартизованого розчину за даними титруванн€ провод€ть, виход€чи з принципу екв≥валентност≥ c н (’)∙ V (X) = c н (Y)Ј V (Y). Ќа основ≥ ц≥Їњ р≥вност≥ обчислюють точну мол€рну концентрац≥ю екв≥валента стандартизованого розчину:
c н(’) =  .
.
ѕриклад 2. ѕри приготуванн≥ приблизно 0,2 н. розчину основи Ba(OH)2 наважку розчинили в м≥рн≥й колб≥ обТЇмом 500 см3 ≥ довели обТЇм колби дистильованою водою до м≥тки. ƒл€ визначенн€ точноњ нормальноњ концентрац≥њ 30,25 см3 цього розчину в≥дтитрували 46,25 см3 робочого розчину 0,1280 н. HCl. ¬изначити масу наважки основи та точну нормальну концентрац≥ю розчину ¬а(ќЌ)2.
–озв ۥ €занн€:
1. m (наважки Ba(OH)2) −?
m (Ba(OH)2) = M (1/2 Ba(OH)2) Ј c (1/2 Ba(OH)2)Ј V (Ba(OH)2Ј10−3 = 85,663Ј0,2Ј500Ј10−3 = 8,5663 г.
2. c н (Ba(OH)2) точна −?
c н (Ba(OH)2) =  =
=  =
=  = 0,1957 M.
= 0,1957 M.
¬≥дпов≥дь: маса наважки ¬а(ќЌ)2 дор≥внюЇ 8,5663 г, а точна концентрац≥€ його розчину складаЇ 0,1957 ћ. 
ќбчисленн€ результат≥в анал≥зу
ѕриклад 3. ќбчислити масову частку Na2CO3 в техн≥чн≥й сод≥, €кщо њњ наважку масою 0,2840 г розчинили в м≥рн≥й колб≥ м≥стк≥стю 100 см3, а середнЇ значенн€ обТЇм≥в 0,1000 ћ розчину HCl, що витратили на титруванн€ ал≥квот обТЇмом 10,00 см3 склало 4,85 см3. “итруванн€ проводили за ≥ндикатором метиловим померанчовим.
–озв ۥ €занн€:
1. f екв(Na2CO3) −? ћ (1/2Na2CO3) −?
«апишемо р≥вн€нн€ реакц≥њ взаЇмод≥њ натр≥й карбонату з хлоридною кислотою:
Na2CO3 + 2HCl Ѓ 2NaCl + H2O + CO2.
як видно з наведеного р≥вн€нн€, один моль Na2CO3 взаЇмод≥Ї з двома мол€ми HCl, кожна молекула Na2CO3 реагуЇ з двома iонами г≥дрогену, тому ≈ (Na2CO3) = 1/2 моль ≥ f екв(Na2CO3) дор≥внюЇ 1/2.
|
|
|
ћ (1/2Na2CO3) = 1/2Ј M (Na2CO3) = 52,995 г/моль.
2. w(Na2CO3) в техн≥чн≥й сод≥ −?
w(Na2CO3) =  Ј100 % =
Ј100 % =
 Ј100 % = 90,5 %.
Ј100 % = 90,5 %.
¬≥дпов≥дь: масова частка Na2CO3 в техн≥чн≥й сод≥ складаЇ 90,5 %.
ѕриклад 4. ќбчислити масову частку основного компоненту Na2B4O7Ј10H2O в зразку бури, €кщо на титруванн€ наважки бури масою 0,8530 г витратили 19,83 см3 0,2100 ћ розчину HNO3.
–озв ۥ €занн€:
1. f екв(Na2B4O7Ј10H2O) −? M≈ (Na2B4O7Ј10H2O) −?
«апишемо р≥вн€нн€ реакц≥њ взаЇмод≥њ натр≥й карбонату з хлоридною кислотою:
Na2B4O7Ј10H2O + 2HNO3 Ѓ 2NaNO3 + 5H2O + 4Ќ3¬O3.
f екв(Na2B4O7Ј10H2O) = 1/2, f екв(Na2CO3) дор≥внюЇ 1/2.
M≈ (Na2B4O7Ј10H2O) = 1/2 M (Na2B4O7Ј10H2O) = 190,71 г/моль.
2. nекв(HNO3), що вступили в реакц≥ю −?
nекв(HNO3) = с (HNO3)Ј V (HNO3)Ј10−3 = 0,2100Ј19,83Ј10−3 = 0,004164 моль.
3. m (Na2B4O7Ј10H2O), що вступила в реакц≥ю −?
nекв(HNO3) = nекв(Na2B4O7Ј10H2O).
m (Na2B4O7Ј10H2O) = nекв(Na2B4O7Ј10H2O)Ј ћ≈ (Na2B4O7Ј10H2O) =
0,004164Ј190,71 = 0,7941 г.
4. w(
Na2B4O7Ј10H2O) −?
w(
Na2B4O7Ј10H2O) = 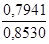 Ј100 % = 93,11 %.
Ј100 % = 93,11 %.
¬≥дпов≥дь: масова частка Na2B4O7Ј10H2O в зразку бури складаЇ 93,11 %.






