«аконы термодинамики и их применение к простейшим процессам. ѕервый закон термодинамики. ¬нутренн€€ энерги€, энтальпи€, теплота, работа. –абота и теплота как характеристики процесса. ¬заимосв€зь теплоты, работы и изменени€ внутренней энергии. ѕрименение первого закона термодинамики к простейшим процессам. “епловые эффекты и теплоты образовани€. —в€зь величины Qр и QV. «акон √есса и его термодинамическое обоснование. “еплоемкость вещества. «ависимость теплоемкости вещества от температуры. «ависимость тепловых эффектов процессов от температуры. ¬ывод и анализ уравнени€ ирхгофа. –асчеты тепловых эффектов химических реакций, теплоты агрегатных и фазовых превращений, теплоты растворени€ при различных температурах.
¬торой закон термодинамики. —амопроизвольные и несамопроизвольные процессы. Ёнтропи€. ѕриведенна€ теплота и энтропи€ дл€ равновесных и неравновесных процессов. »зменение энтропии как критери€ равновеси€ и направлени€ процессов в изолированной системе. ¬ычисление изменени€ энтропии при охлаждении (нагревании) веществ и при фазовых переходах.
“ретий закон термодинамики. ‘ормулировки третьего закона термодинамики и следстви€. ѕостулат ѕланка. ¬ычисление абсолютных стандартных величин энтропии вещества по термодинамическим данным. Ёнерги€ √иббса, энерги€ √ельмгольца, химический потенциал. ‘изический смысл этих величин. ѕрименение энергии √иббса и энергии √ельмгольца в качестве критериев направленности процессов и равновеси€ в изотермических системах. «ависимость энергии √иббса от температуры и давлени€.
’имическое равновесие. ƒинамическа€ и термодинамическа€ характеристика равновеси€. “ермодинамический закон действи€ масс. онстанта равновеси€. —пособы выражени€ константы равновеси€ в гомогенных и гетерогенных системах. ¬ычисление состава равновесной системы, выхода продукта, степени превращени€ исходных веществ, степени диссоциации. ”равнени€ изобары и изохоры химической реакции. ”равнение изотермы и направление химической реакции. онстанта равновеси€ и стандартное изменение энергии √иббса. –асчет изменени€ энергии √иббса и констант равновеси€ дл€ различных температур, приближенные методы расчета константы равновеси€.
«адание 1.1. ќпределение тепловых эффектов химических реакций
¬ычислите тепловой эффект образовани€ вещества ј из простых веществ при температуре 298 и стандартном давлении, если известна его энтальпи€ сгорани€ при этой температуре и стандартном давлении (табл. 1). ѕри вычислении следует прин€ть, что конечные продукты сгорани€ —ќ2(г), Ќ2ќ(ж), N2(г).
“еплоты сгорани€ простых веществ указаны в следующих уравнени€х:
—(графит) + ќ2(г) = —ќ2(г) + 393,51 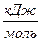 ;
;
Ќ2(г) + 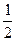 ќ2(г) = Ќ2ќ(ж) + 285,83
ќ2(г) = Ќ2ќ(ж) + 285,83 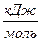 .
.
“аблица 1
| Ќомер варианта | ¬ещество ј | ‘ормула | —осто€ние | ∆ Ќсгор, 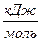
|
| ћочевина | —Ќ4N2ќ | т | Ц632,20 | |
| Ќитрометан | —Ќ3Nќ2 | ж | Ц708,77 | |
| јцетальдегид | —2Ќ4ќ | г | Ц1193,07 | |
| Ётилацетат | —4Ќ8ќ2 | ж | Ц2246,39 | |
| √лицерин | —3Ќ8ќ3 | ж | Ц1661,05 | |
| ƒиметиламин | —2Ќ7N | ж | Ц1768,59 | |
| јцетон | —3Ќ6ќ | ж | Ц1785, 73 | |
| ѕентан | —5Ќ12 | г | Ц3536,15 | |
| “риметиламин | —3Ќ9N | г | Ц2442,92 | |
| ѕропанол-1 | —3Ќ8ќ | ж | Ц2010,41 | |
| Ѕутан | —4Ќ10 | г | Ц2877,13 | |
| јмиловый спирт | —5Ќ12ќ | ж | Ц3320,84 | |
| ‘енол | —6Ќ6ќ | т | Ц3063,52 | |
| √идрохинон | —6Ќ6ќ2 | т | Ц2860,60 | |
| јнилин | —6Ќ7N | ж | Ц3396,20 | |
| Ѕензойна€ кислота | —7Ќ6ќ2 | т | Ц3226,70 | |
| ѕиридин | —5Ќ5N | ж | Ц2755,16 | |
| —теаринова€ кислота | —18Ќ36ќ2 | т | Ц11274,60 | |
| “олуол | —7Ќ8 | ж | Ц3947,94 | |
| ќктан | —8Ќ18 | ж | Ц5470,58 | |
| Ќафталин | —10Ќ8 | т | Ц5156,78 | |
| √ексан | —6Ќ14 | г | Ц4194,75 | |
| »зобутан | —4Ќ10 | г | Ц2868,76 | |
| —ахароза | —12Ќ22ќ11 | т | Ц5646,73 | |
| ”ксусна€ кислота | —2Ќ4ќ2 | ж | Ц874,58 | |
| амфора | —10Ќ16ќ | т | Ц5924,84 | |
| Ѕутанол | —4Ќ10ќ | ж | Ц2671,90 | |
| ‘ормальдегид | —Ќ2ќ | г | Ц561,07 | |
| ўавелева€ кислота | —2Ќ2ќ4 | т | Ц251,88 | |
| јнтрацен | —14Ќ10 | т | Ц7067,45 |
«адание 1.2. ќпределение возможности протекани€ химической реакции в стандартных и нестандартных услови€х
|
|
|
¬ыполните следующие дев€ть заданий дл€ данной реакции (табл. 2) (справочные величины, необходимые дл€ расчетов, возьмите в прил. 1).
1. ѕо значени€м стандартных энтальпий образовани€ участвующих в реакции веществ D H 0 f ,298, вычислите тепловой эффект реакции при стандартных услови€х D H 0298. ¬ыдел€етс€ или поглощаетс€ тепло при протекании реакции? Ёндо - или экзотермической €вл€етс€ данна€ реакци€?
2. ƒл€ исходных веществ и продуктов реакции определите величины средних теплоемкостей 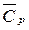 в диапазоне температур 298 Ц “ и изменение теплоемкости Δ
в диапазоне температур 298 Ц “ и изменение теплоемкости Δ 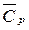 в ходе реакции. ѕользу€сь законом ирхгофа вычислите тепловой эффект реакции D H 0 “ при температуре T и стандартном давлении с учетом величины Δ
в ходе реакции. ѕользу€сь законом ирхгофа вычислите тепловой эффект реакции D H 0 “ при температуре T и стандартном давлении с учетом величины Δ 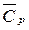 .
.
3. ¬ыведите дл€ реакции функциональную зависимость изменени€ мол€рной изобарной теплоемкости от температуры: D C 0 p = f (T).
4. ¬ычислите величину D H 0 “, пользу€сь законом ирхгофа и установленной функциональной зависимостью D C 0 p = f (T). ак вли€ет увеличение температуры на величину теплового эффекта реакции?
5. ачественно оцените знак изменени€ энтропии D S при протекании реакции. ќбъ€сните полученный результат. ѕо значени€м стандартных энтропий участвующих в реакции веществ S 0298 вычислите изменение энтропии реакции при стандартных услови€х D S 0298.
6. »спользу€ функциональную зависимость D C 0 P = f (T) вычислите изменение энтропии реакции D S 0 T при температуре T и стандартном давлении. ак вли€ет повышение температуры на величину D S 0 T?
7. ачественнооцените веро€тность самопроизвольного протекани€ реакции при высоких и низких температурах. ¬ычислите изменение энергии √иббса D G 0298 реакции, протекающей при стандартных услови€х. ¬озможно ли самопроизвольное протекание процесса при стандартных услови€х? ќпределите температуру T 0, при которой реакци€ мен€ет свое направление.
8. ¬ычислите изменение энергии √иббса D G 0 T реакции, протекающей при стандартном давлении и температуре T, счита€, что D H 0 T и D S 0 T не завис€т от температуры (метод ”лиха). ѕостройте график зависимости D G 0 T от температуры. —делайте вывод о вли€нии температуры на веро€тность самопроизвольного протекани€ процесса в пр€мом направлении.
|
|
|
9. ¬ычислите изменение энергии √иббса D G 0 T реакции при температуре T и стандартном давлении, учитыва€ зависимость D H 0 T и D S 0 T от температуры. —равните полученные значени€ D G 0 T с величиной изменени€ энергии √иббса, рассчитанной по методу ”лиха, и оцените их расхождение.
“аблица 2
| Ќомер варианта | –еакци€ | “, |
| 4NH3(г) + 5O2(г) = 4NO(г) + 6H2O(г) | ||
| 4HCl(г) + O2(г) = 2Cl2(г) + 2H2O(г) | ||
| 2KOH(т) + CO2(г) = K2CO3(т) + H2O(г) | ||
| 2NaHCO3(т) = Na2CO3(т) + H2O(г) + CO2(г) | ||
| 2CuS(т) + 3O2(г) = 2CuO(т) + 2SO2(г) | ||
| Fe3O4(т) + 4H2(г) = 3Fe(т) + 4H2O(г) | ||
| 2H2S(г) + 3O2(г) = 2SO2(г) + 2H2O(г) | ||
| Mg(OH)2(т) = MgO(т) + H2O(г) | ||
| CS2(г) + 3O2(г) = CO2(г) + 2SO2(г) | ||
| MgCO3(т) = MgO(т) + CO2(г) | ||
| Fe2O3(т) + 3H2(г) = 2Fe(т) + 3H2O(г) | ||
| Ca(OH)2(т) + CO2(г) = CaCO3(т) + H2O(г) | ||
| BaO(т) + CO2(г) = BaCO3(т) | ||
| 4HBr(г) + O2(г) = 2Br2(г) + 2H2O(г) | ||
| 4NH3(г) + 3O2(г) = 2N2(г) + 6H2O(г) | ||
| SnO2(т) + 2H2(г) = Sn(т) + 2H2O(г) | ||
| 4FeS2(т) + 11O2(г) = 2Fe2O3(т) + 8SO2(г) | ||
| 2H2S(г) + O2(г) = 2H2O(г) + S2(г) | ||
| FeO(т) + H2(г) = Fe(т) + H≠2O(г) | ||
| CaO(т) + H2O(г) = Ca(OH)2(т) | ||
| Al2O3(т) + 3SO3(г) = Al2(SO4)3(т) | ||
| 2NO(г) + O2(г) = 2NO2(г) | ||
| 2NiS(т) + 3O2(г) = 2NiO(т) + 2SO2(г) | ||
| TiO2(т) + 2H2(г) = Ti(т) + 2H2O(г) | ||
| PbO(т) + SO3(г) = PbSO4(т) | ||
| 4CO(г) + 2SO2(г) = 4CO2(г) + S2(г) | ||
| CO(г) + H2O(г) = CO2(г) + H2(г) | ||
| Fe2O3(т) + 3CO(г) = 2Fe(т) + 3CO2(г) | ||
| SnO2(т) + 2CO(г) = Sn(т) + 2CO2(г) | ||
| 2ZnS(т) + 3O2(г) = 2ZnO(т) + 2SO2(г) |
«адание 1.3. ’имическое равновесие
√азообразные вещества ј и Ѕ реагируют с образованием газообразного вещества ¬ (табл. 3).
¬ыполните следующие задани€ дл€ данной реакции:
1. ¬ыразите константы равновеси€ KP и KC через равновесное количество вещества ¬, равное x, если исходные вещества ј и Ѕ вз€ты в стехиометрических количествах при общем давлении в системе P и температуре T, .
2. –ассчитайте KP и KC при 300 , если – = 7,5Ј104 ѕа, x = 0,45.
3. ¬ычислите равновесное количество вещества — при давлении в системе, равном 3Ј104 ѕа и температуре 300 .
4. –ассчитайте степень превращени€ веществ ј и Ѕ при T = 300 .
“аблица 3
| Ќомер варианта | ”равнение реакции | Ќомер варианта | ”равнение реакции |
| ј + Ѕ = 1/2¬ | 3/2ј + Ѕ = 3/2¬ | ||
| 1/2ј + Ѕ = ¬ | ј + 3Ѕ = 3¬ | ||
| 3ј + Ѕ = 2¬ | 3ј + Ѕ = ¬ | ||
| 2ј + 3Ѕ = 3¬ | ј + 2Ѕ = 2¬ | ||
| 2ј + 1/2Ѕ = 2¬ | 2ј + 2Ѕ = 1/2¬ | ||
| 3ј + 1/2Ѕ = ¬ | ј + Ѕ = ¬ | ||
| ј + 2Ѕ = ¬ | 2ј + 2Ѕ = ¬ | ||
| ј + Ѕ = 3¬ | 2ј + 2Ѕ = 3¬ | ||
| 1/2ј + Ѕ = 2¬ | 3ј + 3Ѕ = 2¬ | ||
| 1/2ј + Ѕ = 3¬ | 1/2ј + Ѕ = 1/2¬ | ||
| 2ј + 1/2Ѕ = 3¬ | 1/2ј + 1/2Ѕ = 2¬ | ||
| 2ј + 3Ѕ = 2¬ | ј + Ѕ = 3/2¬ | ||
| 3ј + 1/2Ѕ = 3¬ | 2ј + 2Ѕ = 3/2¬ | ||
| 3ј + 1/2Ѕ = 2¬ | 3/2ј + 1/2Ѕ = ¬ | ||
| 1/2ј + 1/2Ѕ = 3¬ | 3/2ј + Ѕ = 3/2¬ |
ѕример 1.1. »стинна€ мол€рна€ теплоемкость серебра в интервале температур от 273 до 1234 выражаетс€ уравнением (см. прил. 1):
— 0 P = 23,97 + 5,27×10Ц3 “ Ц 0,25×105 “ Ц2 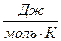 .
.
¬ычислите среднюю мол€рную теплоемкость серебра 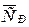 в интервале от 298 до 700 .
в интервале от 298 до 700 .
–ешение. —реднюю теплоемкость в данном интервале температур рассчитывают по уравнению:
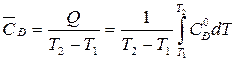 ,
,
где Q Ц количество теплоты, подведенной к системе.
— учетом зависимости истинной теплоемкости от температуры
|
|
|
—P = а + bT + c1 . T Ц2
получим выражение:
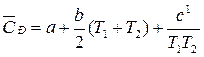 .
.
ѕодставл€€ коэффициенты a, b, c1 и температуру из услови€ задачи, получаем: 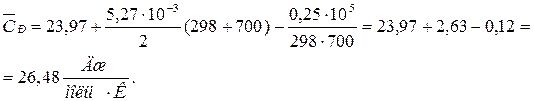
ѕример 1.2. ќпределите энтальпию образовани€ метана, если его энтальпи€ сгорани€ (D H 0 сгор.) равна Ц890,31 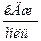 . «начени€ энтальпий сгорани€ водорода и углерода возьмите из термохимических уравнений:
. «начени€ энтальпий сгорани€ водорода и углерода возьмите из термохимических уравнений:
—(графит) + ќ2(г) = —ќ2(г) + 393,51 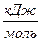 ;
;
Ќ2(г) + 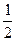 ќ2(г) = Ќ2ќ(ж) + 285,83
ќ2(г) = Ќ2ќ(ж) + 285,83 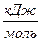 .
.
–ешение. «апишем уравнение реакции образовани€ одного мол€ метана:
—(графит) + 2H2(г) = CH4(г).
“епловой эффект реакции равен
D H 0 = å n D H 0 сгор . (исх.) Ц å n D H 0 сгор . (прод.).
D H 0 = Ц 393,51 + 2∙(Ц285,83) Ц (Ц890,31) = Ц74,86 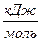 .
.
ѕример 1.3. ¬ыведите функциональную зависимость изменени€ мол€рной изобарной теплоемкости от температуры D C 0 – = f (T) дл€ реакции —(графит) + CO2(г) = 2CO(г). ¬ычислите тепловой эффект реакции при 500 , если при стандартных услови€х он равен 172,5 кƒж.
–ешение. «ависимость C 0 – реагирующих веществ от температуры представлена дл€ неорганических веществ уравнени€ми вида
C 0 – = a + bT + c1T Ц2,
поэтому величину D C 0 – рассчитывают по уравнению
D C 0 – = D a + D bT +D c1T Ц2.
¬ыпишем температурные коэффициенты в уравнени€х теплоемкости дл€ веществ, участвующих в реакции (см. прил. 1).
| ¬ещество | оэффициенты уравнени€ — 0 P = f (T), 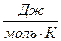
| ||
| а | b ×103 | с1 ×10Ц5 | |
| —(графит) | 16,86 | 4,77 | Ц8,54 |
| —ќ (г) | 28,41 | 4,10 | Ц0,46 |
| —ќ2 (г) | 44,14 | 9,04 | Ц8,53 |
¬ычислим их изменени€:
D a = 2. 28,41 Ц 44,14 Ц 16,86 = -4,18;
D b = (2. 4,10 Ц 9,04 Ц 4,77).10Ц3 = -5,61.10Ц3;
D c1 = [2. (Ц0,46) Ц (-8,53) Ц (-8,54)] .105 = 16,15.105.
‘ункциональна€ зависимость изменени€ мол€рной изобарной теплоемкости от температуры дл€ данной реакции имеет вид:
D C 0 – = -4,18 -5,61.10Ц3 T + 16,15.105 T Ц2.
ƒл€ расчета теплового эффекта реакции воспользуемс€ уравнением ирхгофа в интегральной форме:
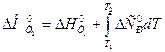 .
.
ѕосле подстановки зависимости D C 0 p от T в уравнение ирхгофа и интегрировани€ получим:
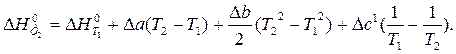
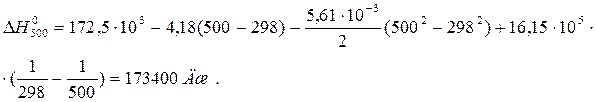
ѕример 1.4. »зменение энтропии реакции —(графит) + CO2(г) = 2CO(г) при стандартных услови€х (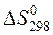 ) равно 175,46
) равно 175,46 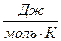 ). »спользу€ функциональную зависимость теплоемкостей реагирующих веществ от температуры (см. пример 1.3), вычислите изменение энтропии при температуре 500 (
). »спользу€ функциональную зависимость теплоемкостей реагирующих веществ от температуры (см. пример 1.3), вычислите изменение энтропии при температуре 500 (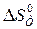 ).
).
–ешение. »зменение энтропии реакции при заданной температуре находим по уравнению
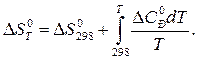
ѕосле подстановки D C 0 – = D a + D bT +D c1T Ц2 и интегрировани€ получим:
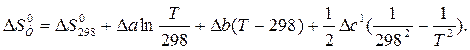
«начени€ D a, D b и D c1 рассчитаны в примере 1.3. ќтсюда:
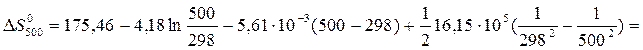 = 178,03
= 178,03 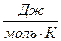 .
.
ѕример 1.5. онстанта равновеси€ KP газовой реакции ј + Ѕ = ¬ равна 0,128Ј105 ѕаЦ1. ќпределите состав равновесной смеси (в мол. %), полученной при давлении – = 10,13Ј105 ѕа из 2 моль вещества ј и 1 моль вещества Ѕ.
–ешение. ќбозначим через x равновесное количество вещества ¬. “ак как на образование его должно израсходоватьс€ согласно химическому уравнению реакции по x молей ј и Ѕ, то в равновесной смеси останетс€ (2 Ц x) моль ј и (1 Ц x) моль Ѕ. «апишем количество вещества
| число молей: | ј + Ѕ = ¬ | ||
| в исходной смеси | |||
| в равновесной смеси | (2 Ц x) | (1 Ц x) | x |
∑ ni = 2 Ц x + 1 Ц x + x = 3 Ц x.
ѕарциальные давлени€ компонентов Pi рассчитываем по уравнению:
Pi = Xi Ј P,
где Xi Ц мольна€ дол€ i -го компонента газовой смеси, Xi = 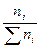 ;
;
P Ц общее давление в системе.
¬ыражени€ дл€ парциальных давлений компонентов имеют вид:
P A = 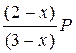 ; P Ѕ =
; P Ѕ = 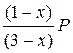 ; P ¬ =
; P ¬ = 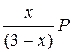 .
.
ѕодставим эти выражени€ в уравнение дл€ константы равновеси€ реакции:
KP = 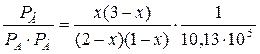 = 0,128Ј105 ѕаЦ1.
= 0,128Ј105 ѕаЦ1.
ѕосле небольших преобразований получим:
2,32 x 2 Ц 6,96 x + 2,64 = 0.
–еша€ квадратное уравнение, находим x 1 = 0,44 и x 2 = 2,55.
|
|
|
¬торой корень не имеет физического смысла, т.к. x может быть только меньше 1. ќстаетс€ значение x = 0,44. —ледовательно, в равновесной смеси содержитс€ 2 Ц 0,44 = 1,56 моль ј; 1 Ц 0,44 = 0,56 моль Ѕ и 0,44 моль ¬. —остав смеси в мольных процентах определ€етс€ из соотношени€
Xi = 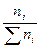 Ј100%,
Ј100%,
где ∑ ni = 1,56 + 0,56 + 0,44 = 2,56.
ќпределим состав равновесной смеси:
X A = 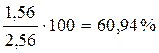 ;
;
X Ѕ = 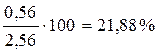 ;
;
X ¬ = 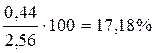 .
.






