Al3+, Zn2+, Cr3+, Sn2+, Sn (IV), As(III), As (V)
Ётапы исследовани€
1. ¬ы€вление As (III), As (V) ионов в отдельной пробе действием металлического цинка в среде HCl:
As(III), (V) Zn, ЌCl AsH3↑
Ѕумага, пропитанна€ AgNO3 AsH3↑ Ag↓ (черный)
(реакци€ √утцайта)
Ѕумага, пропитанна€ [HgCl2] AsH3↑ AsH2(HgCl)↓
(–еакци€ «ангер-Ѕлека) AsH(HgCl)2↓
As(HgCl)3↓
As2Hg3↓
(желто-коричневый)
2. ƒействие избытка 6 моль/дм3 NaOH в присутствии Ќ2ќ2 на катионы IV аналитической группы при нагревании:
Al3+ NaOH Al(OH)3↓ избыток NaOH [Al(OH)6]3ˉ
Zn2+ NaOH Zn(OH)2↓ избыток NaOH [Zn(OH)4]2ˉ
Cr3+ NaOH Cr(OH)3↓ избыток NaOH,H2O2,Δ CrO2ˉ4
Sn2+ NaOH Sn(OH)2↓ избыток NaOH, H2O2, Δ [Sn(OH)6]2ˉ
Sn(IV) NaOH Sn(OH)4↓ избыток NaOH [Sn(OH)6]2ˉ
As(III) NaOH AsO3ˉ3 H2O2, Δ AsO3ˉ4
As(V) NaOH AsO3ˉ4 H2O2, Δ AsO3ˉ4
3. ќтделение гидроксоанионов [Al(OH)6]3ˉ; [Sn(OH)6]2ˉ из раствора є2 действием кристаллического NH4Cl при нагревании:
[Al(OH)6]3ˉ NH4Cl, Δ Al(OH)3↓
[Sn(OH)6]2ˉ NH4Cl,Δ Sn(OH)4↓
4. –астворение осадка є3 действием 2 моль/дм3 HCl:
Al(OH)3↓ HCl Al3+
Sn(OH)4↓ HCl [SnCl6]2ˉ
5. ¬ы€вление Al3+ катиона действием на раствор є4 ализарина или натри€ ацетата:
Al3+ ализарин, NaOH 
Al3+ CH3COONa Al(OH)2CH3COO↓
6. ¬ы€вление Sn(IV) действием раствора соли меркурий (II) Ц катионов на прокип€ченный с железными в среде HCl раствор є4:
[SnCl6]2ˉ Fe, HCl; Δ Sn2+ HgCl2 Hg↓
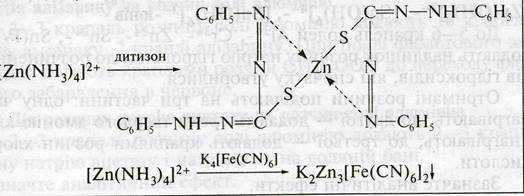
7. ¬ы€вление Zn2+ - катиона в центрифугате є3 действием раствора дитизона, или K4[Fe(CN)6]:
ƒействие некоторых реагентов на катионы V аналитической группы Fe2+, Fe3+, Mg2+, Mn2+
| –еагент | атионы | |||||||||
| Fe2+ | Fe3+ | Mg2+ | Mn2+ | |||||||
| NaOH; KOH | Fe(OH)2 зеленый осадок | Fe(OH)3 красно-бурый осадок | Mg(OH)2 белый осадок | Mn(OH)2 белый осадок, который становитс€ бурым на воздухе вследствие образовани€ MnO * nH2O | ||||||
| ќсадки раствор€ютс€ в кислотах и в растворе NH4Cl (кроме Fe(OH)3) | ||||||||||
| NH4OH | Fe(OH)2 зеленый осадок | Fe(OH)3 красно-бурый осадок | Mg(OH)2 белый осадок | Mn(OH)2 белый осадок, который становитс€ бурым на воздухе вследст образовани€ MnO * nH2O | ||||||
| Na2HPO4 + NH4OH | Fe3 (PO4)2 | FePO4 | MgNH4PO4 | Mn3 (PO4)2 | ||||||
| Ѕелые осадки, раствор€ютс€ в минеральных кислотах, не раствор€ютс€ в уксусной кислоте | ||||||||||
| HNO3, H2O2 | - | - | - | - | ||||||
ƒействие некоторых реагентов на катионы V аналитической группы Bi3+ , Sb3+, Sb5+
| –еагент | атионы | ||||||
| Bi3+ | [SbCl6]3ˉ | [SbCl6]ˉ | |||||
| NaOH; KOH | Bi(OH)3 белый осадок | Sb(OH)3 белый осадок | HSbO3 белый осадок | ||||
| –аствор€ютс€ в HCl | |||||||
| –аствор€ютс€ в щелочах | |||||||
| NH4OH | BiONO3 или BiOCl белый осадок, раствор€етс€ в кислотах | Sb(OH)3 белый осадок | HSbO3 белый осадок | ||||
| Na2HPO4 + NH4OH | BiPO4 белый осадок, не раст-с€ в уксусной к-те и разведенной азотной кислоте | Ѕелые осадки основных солей переменного состава | |||||
| HNO3, H2O2 | HSbO3 Ѕелые осадки, которые раствор€ютс€ в HCl и щелочах | ||||||
|
|
|






