1. ѕолучена смесь азота и водорода и установлены услови€ реакции. –авновесие в системе:
2 6 4 (вступило в реакцию и образовалось)
N2 + 3H2 ↔ 2NH3
1 2 4 (концентрации в равновесии)
- установилось при следующих концентраци€х веществ
[N2]= 1 моль\л
[H2]=2 моль\л
[NH3]=4 моль\л
¬ычислите исходные концентрации N2 и H2, а также константу химического
равновеси€
ќтвет: 
[N2]исходна€ = 1 моль\л +2 моль\л = 3 моль\л
[H2]исходна€ = 2 моль\л +6 моль\л = 8 моль\л
2. онцентраци€ хлорноватистой кислоты (HClO) в водном растворе равна 0,001 моль\л.
Х ¬ычислите степень диссоциации кислоты, концентрацию ионов водорода и рЌ раствора, если константа диссоциации кислоты дисс=10-7
α=  = 10-2 или 1%. рЌ= -lg
= 10-2 или 1%. рЌ= -lg  = 5. [H+] =
= 5. [H+] = 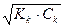 = 10-5
= 10-5
2а. ќпределите степень диссоциации, рЌ среды и концентрацию протонов в водном растворе NH4OH, концентраци€ которого равна 0,001 моль\л, дисс=10-5
α=  = 10-1 или 10%. рќЌ= -lg
= 10-1 или 10%. рќЌ= -lg  = 4.
= 4.
[ќH-] =  = 10-4
= 10-4
рЌ = 14 Ц рќЌ = 14 -4 =10 [H+] = 10-10 моль/л
3. ¬ычислить DЌ реакции:
N2H4 + O2 = N2 + 2H2O
(газ) (газ) (газ) (газ)
если DЌ0обр, кƒж\моль, N2H4(г)= -80 H2O(г)= -242
ќпределите знак DS реакции, и может ли быть осуществлена эта реакци€ в стандартных услови€х (возможность самопроизвольного протекани€)
DrH0298 = ∑ v i Ј D f H0298, i - ∑ vj ЈD f H0298, j = 0 + 2(-242) Ц (-80 + 0) =-404 кƒж
прод. исход.
где DrH0298 Ч тепловой эффект химической реакции
DS реакции больше нул€ (DrS>0), так как количество газовых молекул до реакции равно 2, а после Ц 3, т.е. увеличилось. ќба слагаемых в уравнении D G = D Ќ-“ D S отрицательны при любых температурах и Dr G <0, поэтому возможно самопроизвольное протекание пр€мой реакции
4. ќпределите осмотическое давление раствора в 500 мл которого содержитс€ 4,5г фруктозы C6H12O6 (R = 0,082 л.атм/град.моль)
»спользуем уравнение ¬ант-√оффа –ќ—ћ. = —ћRT и ли –ќ—ћ. = nRT/V, n =m/M –ассчитываем ћ (C6H12O6) = 6 Ј12 + 12Ј1 + 6Ј16 = 180, V = 0,5 л
ќтвет: –осм. = 1,22 атм.
5. ¬ 250 мл воды растворили 60г мочевины CO(NH2)2. ќпределите температуру замерзани€ полученного раствора, если криоскопическа€ посто€нна€ воды =1,86.
»спользуем закон –аул€: Δ“зам. = “. —m,
где Δ“зам. Ц понижение температуры замерзани€ раствора по сравнению с температурой замерзани€ чистого растворител€, —m Ц мол€льна€ концентраци€ раствора, “ Ц криоскопическа€ посто€нна€
 ћол€льна€ концентраци€ Ц это химическое количество вещества, растворенное в одном килограмме растворител€:
ћол€льна€ концентраци€ Ц это химическое количество вещества, растворенное в одном килограмме растворител€:
Х Cm = = m/M . mрастворител€ = 4 моль/кг
Δ“зам. = “. —m = 4.1,86 = 7,440
6. ќпределите, в какой среде - кислой, нейтральной или щелочной Ц сильнее про€вл€ютс€ окислительные свойства иона MnO4 -. Ќапишите соответствующие уравнени€ полуреакций восстановлени€ ионов MnO4 Ц и по таблице найдите величины окислительно-восстановительных потенциалов
|
|
|
≈ = ≈0 + (0,059/n) . lg ([Ox]/[Red])
¬ кислых:
KMnO4 + KNO2 + H2SO4 = MnSO4 + K2SO4 + KNO3 + H2O
MnO4- + 8H+ + 5e = Mn2+ + 4H2O 2 восстановление
NO2- + H2O - 2e = NO3- + 2H+ 5 окисление
—ложим первое уравнение полуреакции, умножив на 2, со вторым, умноженным на 5
2MnO4- + 16H+ + 10e +5NO2- + 5H2O - 10e = 2Mn2+ + 8H2O + 5NO3- + 10H+
—окращаем коэффициенты с одинаковыми ионами и электронами:
2MnO4- + 6H+ +5NO2- = 2Mn2+ + 3H2O + 5NO3-
2KMnO4 + 5KNO2 + 3H2SO4 = 2MnSO4 + K2SO4 + 5KNO3 + 3H2O ≈0 MnO4- / Mn2+ = 1,51 ¬
¬ нейтральных:
KMnO4 + K2SO3 + H2O = MnO2 + K2SO4 + ќH
2KMnO4 + 3K2SO3 + H2O = 2MnO2 + 3K2SO4 + 2 ќH ≈0 MnO4- / MnO2 = 0,60 ¬
¬ основных:
а) KMnO4 + K2SO3 + KOH = K2MnO4 + K2SO4 + H2O ≈0 MnO4- / MnO4-2 = 0,56 ¬
б) KMnO4 +—Ќ3ќЌ +KOHэлектронами = 2MnO4+ K2—O3 + H2O
а) 2KMnO4 + K2SO3 + 2KOH = 2K2MnO4 + K2SO4 + H2O
б) 6KMnO4 +—Ќ3ќЌ +8KOHэлектронами =6 2MnO4+ K2—O3 + 6H2O
7. Ќа основании величин стандартных электродных окислительно-восстановительных
потенциалов (таблица),укажите, в каком направлении может самопроизвольно
протекать реакци€:
Cl2+2KF ↔ 2KCl+F2
Cl2+2e- ↔ 2Cl- окислитель восстанавливаетс€
2F- -2e- ↔ F2 восстановитель окисл€етс€
—равним стандартные электродные потенциалы: E Cl2/Cl- = 1,36 B < E F2/F- = 2,87 B. ќкислителем может быть фтор. –еакци€ потечет в обратном направлении
8. Ќа основании концепции гибридизации атомных орбиталей,определите
геометрическую конфигурацию молекул BeCl2, –F3 и ¬F3,пол€рность, типы межмолекул€рного взаимодействи€



Mолекулы BeCl2 и ¬F3 симметричны, значит их суммарный дипольный момент равен нулю и они непол€рны. ћежду молекулами может наблюдатьс€ только дисперсионное взаимодействие

– фосфор (основное состо€ние) –F3, фосфор находитс€ в одной подгруппе с азотом и €вл€етс€ его элементом-аналогом sp3 Ц искаженный тетраэдр, молекула пол€рна, как и молекулы NH3, NF3. ћежду молекулами этих соединений возможно все типы межмолекул€рного взаимодействи€: ориентационное, индукционное и дисперсионное

9. ¬ычислите степень гидролиза ацетата кали€ в 0,001 ћ растворе этой соли, и значение рЌ раствора. Ќапишите уравнение гидролиза. дисс(CH3COOH) = 10-5
CH3COOK + H2O ↔ CH3COOH + KOH
 α=
α=  = = 10-3
= = 10-3
 |
рќЌ = - lg = 6
 |
pH = 14 Ц рќЌ = 14 + lg = 8
10. ќпределить рЌ среды 0,1 ћ растворов солей: 1)NH4Cl, дис. (NH4OH) = 10-5.
2)Na2CO3 (карбонат натри€), дисс1 (Ќ2CO3) = 10-7, дисс2 (Ќ2CO3) = 10-11
—оли сильные электролиты и полностью диссоциируют на ионы
NH4Cl →NH4+ + Cl- соль образована слабым основанием
NH4+ + ЌќЌ ↔ NH4OH + Ќ+ гидролиз по катиону, кисла€ среда
pH = -lg  = = -lg
= = -lg  = 5
= 5
Na2CO3 → 2Na+ + CO32- соль образована слабой кислотой
CO32- + ЌќЌ ↔ ЌCO3- + ќЌ- перва€ стади€ гидролиза по аниону которой соответствует константа гидролиза равна€ частному от делени€ ионного произведени€ воды на константу диссоциации второй стадии угольной кислоты:
 | |||
 | |||
г1 = α1г = 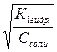 = = 10-1= 0,1
= = 10-1= 0,1
ЌCO3- + ЌќЌ ↔ Ќ2CO3 + ќЌ- втора€ стади€ гидролиза по аниону которой соответствует константа гидролиза равна€ частному от делени€ ионного произведени€ воды на константу диссоциации первой стадии угольной кислоты:
|
|
|
 |
г2 = α2г =  =
=  = 10-4
= 10-4
“ак как константа гидролиза первой стадии в миллион раз больше константы гидролиза второй стадии, а степень гидролиза по первой стадии в тыс€чу раз больше, чем по второй и при расчете рЌ раствора гидролизом по второй стадии можно пренебречь:
 |
Х рќЌ = - lg = 2
 |
pH = 14 Ц рќЌ = 14 + lg =12
11. ќпределите концентрацию ионов ќЌ Ц в насыщенном водном растворе малорастворимого Al(OH)3, если ѕ– (Al(OH)3) = 27∙10-8. ќпределите рЌ раствора.
x x 3x
Al(OH)3 → Al+3 + 3OH-
ѕ– (Al(OH)3)= [Al+3]∙[OH-]3 = x ∙(3x)3 = 27x 4 = 27∙10-8, x = 10-2 , [OH-] = 3∙10-2
рќЌ = 1,52 рЌ = 14 Ц рЌ = 14 Ц 1,52 = 12,48
12. ќпределите знак DS0 дл€ приведенных ниже реакций. — учетом знака DЌ0, указанного
дл€ каждой реакции, оцените, какие из реакций могут протекать самопроизвольно,
и при каких температурах (высоких или низких) это возможно:
a) 2N2 + O2 = 2N2O; DЌ0 >0 ΔS < 0, ΔG > 0, реакци€ невозможна
б) N2 + O2 = 2NO; DЌ0 >0 ΔS = 0, ΔG > 0, реакци€ невозможна
в) 2NO + O2 = 2NO2; DЌ0 <0 ΔS < 0, возможна при низких температурах
г) NO + NO2 = N2O3; DЌ0 <0 ΔS = 0, ΔG < 0 возможна при любой температуре
¬се вещества наход€тс€ в газовой фазе
13. ќпределите мол€рную и нормальную (эквивалентную) концентрацию раствора серной кислоты, в котором массова€ дол€ кислоты составл€ет 35%, а плотность 1,20г/мл.
ћол€рна€ концентраци€ Ц это химическое количество вещества, содержащеес€ в одном литре раствора: —ћ =  . ѕусть Vраствора = 1л, тогда —ћ = n =
. ѕусть Vраствора = 1л, тогда —ћ = n = 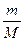 , масса вещества, выраженна€ через массовую долю равна: m = ω.mраствора = ω.ρ.1000, так как ω =
, масса вещества, выраженна€ через массовую долю равна: m = ω.mраствора = ω.ρ.1000, так как ω =  , а масса одного литра раствора равна: mраствора = ρ.1000, где ρ Ц плотность раствора в г/мл. ѕодставл€ем полученное выражение дл€ массы вещества и получаем: —ћ = ω. ρ.1000/M =
, а масса одного литра раствора равна: mраствора = ρ.1000, где ρ Ц плотность раствора в г/мл. ѕодставл€ем полученное выражение дл€ массы вещества и получаем: —ћ = ω. ρ.1000/M =  =
=  моль/л
моль/л
Cн. = Z.CM =8,56 моль.экв./л
14. ƒл€ растворени€ 5,2 г некоторого металла потребовалось 14,7 г серной кислоты (счита€ на чистое вещество). ќпределите эквивалентную массу металла и объем выделившегос€ водорода.
«акон эквивалентов дл€ масс реагирующих веществ: массы реагирующих и образующихс€ относ€тс€ друг к другу, как их эквивалентные массы

ƒл€ расчета объема выделившегос€ водорода используем формулу включающую эквивалентные объемы газов при нормальных услови€х вместо эквивалентных масс
оличество моль.экв. водорода равно химическому количеству эквивалентов серной кислоты и металла. ќбъем выделившегос€ водорода равен произведениюхимического количества эквивалентов на объем моль.экв. водорода:

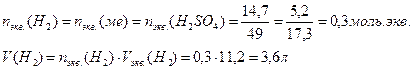
15. ¬одородные соединени€ элементов второго периода.
√идриды металлов - основани€ кислоты
Li+H-, Be+2H2-, B+3H-3, CH4, N-3H3+, H2+O-2, H+F-
Li+H- + N-H3+ = LiNH2 + H2 Li+H- + H2+O-2= LiOH + H2 Na0 + N-H3+ = NaNH2 + H2
N-3H3+ + H+ = [NH4]+ H2+O-2+ H+ = [H3O]+
√идриды металлов могут оторвать протон даже св€занный с углеродом. Ќапример, от α-положени€ к карбонильной группе:
—Ќ3-—ќ-—Ќ3 + Li+H- = Li+ -—Ќ2-—ќ-—Ќ3 + Ќ2
омплексные гидриды используютс€ как восстановители органических соединений:
Li+H- +Al+3H3- = Li+[AlH4]- алюмогидрид лити€
B+3H-3 + Na+H- = Na+[BH4]- натри€ борогидрид
—Ќ3-—ќ-—Ќ3 + Na+[BH4]-→ —Ќ3-—ЌќЌ-—Ќ3
15. Ќапишите уравнени€ реакции диссоциации комплексного соединени€ K2[CoCl4]
в растворе. Ќапишите выражение дл€ константы нестойкости этого комплекса
первична€ диссоциаци€ Ц необратимый распад на внутреннюю и внешнюю сферу: K2[CoCl4] → 2K++[CoCl4]2-
вторична€ диссоциаци€ Ц обратимый распад внутренней сферы. ажда€ ступень характеризуетс€ своей константой нестойкости:
[CoCl4]2- ↔ [CoCl3]-+ Cl- 
|
|
|
[CoCl3]- ↔ [CoCl2]0 + Cl- 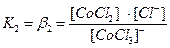
[CoCl2]0 ↔ [CoCl]+ + Cl- 
[CoCl]+ ↔ Co2+ + Cl- 
ќбщие (суммарные) константы нестойкости комплексов характеризуют их полную диссоциацию на комплексообразователь и лиганды и равны произведению ступенчатых констант:
[CoCl4]2- ↔ Co2+ + 4Cl- полна€ вторична€ диссоциаци€

16. ќпределите реакцию среды в водных растворах следующих солей:
3PO4 CuSO4 Ca(NO3)2
ѕодтвердите свой вывод уравнени€ми соответствующих реакций гидролиза.
3PO4 → 3 + + PO4-3
PO4-3 + HOH ↔ HPO4-2 + OH- (основна€ среда)
CuSO4 → Cu+2 + SO4-2
Cu+2 + HOH ↔ (CuOH) + + H+ (кисла€ среда)
Ca(NO3)2 → Ca+2 + 2NO3- (нейтральна€ среда)
17. Ќа какие ионы будут диссоциировать в водном растворе соли, имеющие состав
KAl(SO4)2 и K3AlF6? Ќапишите соответствующие уравнени€ диссоциации
јлюминий переходный металл и его трехзар€дный катион €вл€етс€ комплексообразователем:
KAl(SO4)2 → K+ + [Al(SO4)2]- (первична€ диссоциаци€, SO42- - бидентатный лиганд, координационное число комплексообразовател€ равно 4)
[Al(SO4)2]-↔ Al+3 + 2SO4)2-2 (полна€ вторична€ диссоциаци€)
K3AlF6 → 3K+ + [AlF6]-3 (первична€ диссоциаци€, координационное число Al+3 Ц 6)
[AlF6]-3 ↔ Al+3 + 6F- (полна€ вторична€ диссоциаци€)
18. ”кажите, между молекулами каких соединений может образовыватьс€ водородна€ св€зь (схема образовани€ св€зи):
SiH4, CH3OH, H2S, CH3COOH, HBr, NH3, ¬F3, HF, CH3Cl, H2O, —Ќ3-—ќ-—Ќ3
ћежду молекулами, содержищими Ќ-’ св€зь,где ’=N, ќ, F, Cl, Br, I и т.д., могут образоватьс€ водородные св€зи, в частности, всех кислот, спиртов, аминов
SiH4, CH3OH, H2S, CH3COOH, HBr, NH3, ¬F3, HF, CH3Cl, H2O, —Ќ3-—ќ-—Ќ3
CH3OH, H2S, CH3COOH, HBr, NH3, HF, H2O Ц могут
SiH4, ¬F3, CH3Cl, —Ќ3-—ќ-—Ќ3 Ц не могут
19. ќпределите значение водородного показател€, рЌ, в растворе HCl с концентрацией 0,0001 моль/л и в растворе NaOH с концентрацией 0,001 моль/л
| рЌ —ильной кислоты HCl→ H+ + Cl- | pH = -lg[H+] = -lg—н, где —н или —эк., —N Цнормальна€ концентраци€ кислоты. рЌ =-lg10-4 =4 |
| рЌ —ильного основани€ NaOH→Na+ + OH- | рќЌ = - lg[OH-] = - lg—o, где —o или —эк., —N Ц нормальна€ концентраци€ основани€ рЌ = 14 - рќЌ= 14 + lg[OH-] = 14 + lg—o = 14 -3 = 11 |
20. ќпределите зар€д и координационное число иона-комплексообразовател€ в соединени€х: Mg2[Fe(CN)6], Na2[Co(NH3)2(NO2)4], [Co(NH3)5(H2O)]Br3
CN-, (NH3)0, NO2- , (H2O)0
+2, 6 +2, 6 +3, 6
Mg2[Fe(CN)4 (Nќ2)2], Na2[Co(NH3)2(—N)4], [Co(NH3)5(H2O)]Br3
+4 +2 -4 -2 +2 +2 0 -4 +3 0 0 -3
—уммарный зар€д всех ионов комплексного соединени€ равен нулю
21. Ќе производ€ вычислени€, определите знак изменени€ энтропии в следующих реакци€х:
3H2 (г) + N2(г) = 2NH3 (г)
NH4NO2 (кр) = N2 (г) + 2H2O(г)
Na2CO3(ж) + 2HCl (ж) = 2NaCl(ж) + H2O(ж) + CO2(г)
3H2 (г) + N2(г) = 2NH3 (г) ∆S<0
NH4NO2 (кр) = N2 (г) + 2H2O(г) ∆S>0
Na2CO3(ж) + 2HCl (ж) = 2NaCl(ж) + H2O(ж) + CO2(г) ∆S>0
22. ¬о сколько раз изменитс€ скорость химической реакции (пр€мой)
2NO(газ) + O2(газ) = 2NO2(газ)
если уменьшить объем реакционного сосуда в 2 раза?

23. ”кажите, какие из перечисленных ионов могут быть восстановител€ми:
SO4-2, NO2-, Cu2+, Sn2+, F-, IO4-, Al3+, I-, Fe3+, SO32-
SO4-2, NO2-, Cu2+, Sn2+, F-, JO4-, Al3+, I-, Fe3+, SO32-
SO4-2 (S+6), Cu2+, F-, IO4- (I+7), Al3+, Fe3+ - не могут
NO2- (N+3), Sn2+, I-, SO32- (S+4) Ц могут
24. ќпределите стандартное изменение энтальпии DrЌ0 реакции сгорани€ этана
|
|
|
2C2H6(г) + 7O2(г) = 4CO2(г) + 6H2O (г) и стандартную энтальпию сгорани€
этана DflH0298 по известным энтальпи€м образовани€:
DЌ0(CO2(г)) = -393,5 кƒж\моль
DЌ0(H2O (г)) = -241,8 кƒж\моль
DЌ0(CH4(г)) = -89,7 кƒж\моль
«акон √есса: DrH0298 = ∑ v i Ј D f H0298, i - ∑ vj ЈD f H0298, j=
прод. исход.

—тандартной энтальпией сгорани€ называетс€ энтальпи€ реакции сгорани€ одного мол€ вещества в кислороде с образованием оксидов элементов, имеющих высшую степень окислени€
C2H6(г) + 7/2O2(г) = 2CO2(г) + 3H2O (г)
DflH0298 =DrH0298/2 = -2845,4/2 = -1422,7 кƒж/моль
25. “емпературный коэффициент скорости химической реакции равен 3. ¬о сколько раз возрастет скорость реакции при повышении температуры от 600 — до 900 —?

26. –асставить коэффициенты методом полуреакций и на основании величин стандартных электродных потенциалов (таблица), укажите, какие из приведенных реакций могут самопроизвольно протекать в кислом водном растворе:
1) MnO4- + Cl- + H+ ↔ MnO2 + Cl2 + H2O
2) MnO4- + Br-+ H+ ↔ MnO2 + Br2 + H2O
3) MnO4- +F- + H+ ↔ MnO2 + F2 + H2O
2MnO4- + 6Cl- + 8H+ ↔ 2MnO2 + 3Cl2 + 4H2O может
MnO4- + 4H+ + 3e- ↔ MnO2 + 2H2O E0= 1,70 B
2Cl- - 2e- ↔ Cl2 E0= 1,36 B
2MnO4- + 6Br-+ 8H+ ↔ 2MnO2 + 3Br2 + 4H2O может
MnO4- + 4H+ + 3e- ↔ MnO2 + 2H2O E0= 1,70 B
2Br- - 2e- ↔ Br2 E0= 1,07 B
2MnO4- +6F- + 8H+ ↔ 2MnO2 + 3F2 + 4H2O не может
MnO4- + 4H+ + 3e- ↔ MnO2 + 2H2O E0= 1,70 B
2F- - 2e- ↔ F2 E0= 2,87 B
27. ¬ закрытом сосуде протекает обратима€ химическа€ реакци€:
2NO (газ) + O2 ↔ 2NO2(газ)
Ќачальные концентрации введенных в сосуд веществ составили:
[NO]=10 моль/л
[O2]=12,5 моль/л.
–авновесие наступило после того, как было израсходовано 50% оксида азота (II), NO. ќпределите равновесные концентрации NO, NO2 , O2; вычислите константу равновеси€
5 2,5 израсходовалось
2NO (газ) + O2 ↔ 2NO2(газ)
5 10 5 концентрации в равновесии
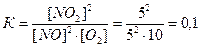
28. —колько грамм 10% сол€ной кислоты необходимо вз€ть, чтобы получить 250 мл 0,1 н раствора? „ему равна мол€рна€ концентраци€ этого раствора?
ω% = 10%, ω=0,1; Vраствора = 250 мл = 0,25 л
—ћ =  .
.
ω = 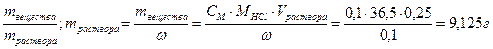
CЌ = Z∙CM; —ћ = —н/ Z = 0,05 моль/л
29. акой объем грамм 50%-ной серной кислоты (плотность ρ =1,4 г/мл) надо вз€ть, чтобы приготовить 2 л 0,1 н раствора этой кислоты? „ему равна мол€рна€ концентраци€ этого раствора?
ω% = 50%, ω=0,5; Vраствора = 2000 мл = 2 л
—ћ =  .
.
 ω =
ω =  г
г
V2раствора= 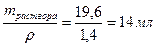
CЌ = Z∙CM; —ћ = —н/ Z = 0,05 моль/л
—одержание
–ј—“¬ќ–џ
ƒисперсные системы






