ƒо сих пор мы предполагали и рассматривали процесс диссоциации рас-
творенного вещества под действием молекул растворител€. Ќо возможен про-
цесс и самодиссоциации растворител€. “о есть взаимодействие между собст-
венными молекулами растворител€ настолько значительно, что может привести
к разрыву св€зей внутри молекул с образованием новых зар€женных частиц Ц
ионов. Ќапример, вода диссоциирует на ионы водорода и гидроксила
Ќ2ќжид. ↔ Ќ+раствор + ќЌ-раствор,
и как следствие Ц чиста€ вода обладает электропроводностью (правда, незначи-
тельной). —ледует отметить, что в воде протон сольватирован и существует в виде иона гидроксони€ (гидрони€) Ќ3ќ+. — учетом этого уравнение процесса диссоциации воды (автопротолиз воды) можно записать:
2Ќ2ќ ↔ Ќ3ќ+ + ќЌ-
Ќа практике, однако, чаще используют форму записи Ќ+, чем Ќ3ќ+
¬ услови€х равновеси€ процесс самодиссоциации воды можно оха-
рактеризовать константой диссоциации н2о
[ H + ] Ј [OH − ]
д (н2о) = ЧЧЧЧЧЧЧ.
[ H 2 O]
≈е величина очень мала и при 22 о— составл€ет значение равное 1,8 Ј10-16.
»з этого следует, что в воде образуетс€ очень незначительное количество ионов
водорода Ќ+ и гидроксила ќЌ- (числитель выражени€), а следовательно, и рас-
падаетс€ незначительна€ часть молекул воды. ѕоэтому можно без большой
ошибки считать равновесную концентрацию нераспавшихс€ молекул воды
[H2O] в приведенном выражении равной концентрации исходной воды (до дис-
социации), котора€ дл€ одного литра воды, принима€ во внимание плотность
воды и ее мол€рную массу, равна:
1000 г
[H2O] = ЧЧЧЧЧЧЧ = 55,56 моль/л
18 (г / моль) Ј 1л
где 1000 г Ц масса одного литра воды, 18 Ц мол€рна€ масса воды. ≈сли подста-
вить величину концентрации воды в выражение константы диссоциации, то по-
лучим:
[ H+][OH−]
1,8Ј10-16 = ЧЧЧЧЧ или [H+][OH-] = воды Ј 55,56 = 1,8 Ј 10-16 Ј 55,56 =
55,56
1Ј10-14.
ѕоследнее выражение ─ произведение мол€рных концентраций ионов водорода и гидроксила ─ называют ионным произведением воды. ¬еличина эта посто€нна€ при
данной температуре и характеризует соотношение концентраций ионов водо-
рода и гидроксила чистой воды. »з ионного произведени€ воды видно, что кон-
центрации ионов водорода и гидроксила равны между собой и в чистой воде
составл€ют величину, равную
[H+] = [OH-] = 1Ј10-7 моль/л.
Ёто и пон€тно, так как кажда€ распавша€с€ молекула воды поставл€ет одно-
временно один ион водорода Ќ+ и один ион гидроксила ќЌ-.
≈сли вспомнить, что ион водорода Ќ+ €вл€етс€ носителем кислотных
свойств, а ион гидроксила ќЌ- ─ основных, то при равенстве их концентраций
[H+] = [OH-] = 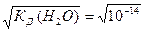 =10-7 моль/л вода €вл€етс€ нейтральным электролитом: ни кислым, ни основным. ¬ода выступает в роли амфолита. ≈сли же концентраци€ ионов водорода [H+] в водном растворе будет в силу каких-то причин больше концентрации ионов гидроксила [OH-] (например, при добавлении молекул кислоты, распадающихс€ на ионы водорода и кислотного остатка), то раствор будет кислым. ≈сли же, наоборот, концентраци€ ионов гидроксила будет больше, чем
=10-7 моль/л вода €вл€етс€ нейтральным электролитом: ни кислым, ни основным. ¬ода выступает в роли амфолита. ≈сли же концентраци€ ионов водорода [H+] в водном растворе будет в силу каких-то причин больше концентрации ионов гидроксила [OH-] (например, при добавлении молекул кислоты, распадающихс€ на ионы водорода и кислотного остатка), то раствор будет кислым. ≈сли же, наоборот, концентраци€ ионов гидроксила будет больше, чем
|
|
|
ионов водорода, то раствор окажетс€ основным.
Ќапример, если в растворе концентраци€ ионов водорода [H+] = 10-4
моль/л, а концентраци€ ионов гидроксила соответственно равна [OH-] =10-10
моль/л (их произведение должно равн€тьс€ [H+][OH-]= 10-14), то концентраци€
ионов водорода больше концентрации ионов гидроксила [H+] > [OH-], и раствор
окажетс€ кислым (говор€т: среда кисла€).
ƒл€ характеристики кислотности среды можно установить различные
шкалы, использу€ соотношение между концентраци€ми ионов водорода и гид-
роксила, как это представлено на рис. 2.2.
а [H+], моль/л 10-1 10-2 10-3 10-4 10-5 10-6 10-7 10-8 10-9 10-10 10-11 10-12 10-13 10-14
кисла€ среда нейтральна€ среда щелочна€ среда
б рЌ=- lg[ H +] 1 2 3 4 5 6 7 8 9 10 11 12 13 14
–ис. 2.2. Ўкалы кислотности с использованием: а Ц концентрации
ионов водорода, моль/л; б Ц водородного показател€ рЌ
»спользовать шкалу Ђаї (–ис.2.2) не совсем удобно, приходитс€ все врем€
выражать концентрацию ионов водорода в моль/л, примен€€ отрицательную
степень. ¬ практической работе прин€то выражать концентрацию ионов водо-
рода через так называемый водородный показатель рЌ, который представл€ет
собой просто один только показатель степени концентрации ионов водорода
без отрицательного знака. — математической точки зрени€ рЌ ─ это отрица-
тельный дес€тичный логарифм мол€рной концентрации ионов водорода:
рЌ = - lg[H+].
≈сли, например, [H+] = 10-4 моль/л, то рЌ = - lg[10-4]= 4 (среда кисла€). ѕри рав-
ных концентраци€х ионов водорода и гидроксила [H+] = [OH-] = 10-7моль/л рас-
твор будет нейтральным и его рЌ = - lg [10-7] = 7 (среда нейтральна€). ≈сли рЌ <
7, то среда кисла€; если рЌ > 7, то среда щелочна€.
»ногда примен€ют не показатель рЌ, а показатель ионов гидроксила рќЌ
(по аналогии с рЌ), равный
рќЌ = -lg[OH-].
—умма показателей ионов водорода и гидроксила дл€ данного раствора должна
быть равной 14, т.е. рЌ + рќЌ = 14, так как логарифмирование ионного произ-
ведени€ воды дает следующее соотношение lg ([H+]Ј[OH-]) = lg 10-14, или lg[H+] + lg[OH-] = -14, а отсюда, принима€ во внимание, что рЌ = -lg[H+] и pOH = - lg[OH-], получим:
рЌ + рќЌ = 14.
—ледует отметить, что при расчете рЌ и рќЌ концентраци€ми [H+] и [OH-] можно пользоватьс€ лишь в растворах, ионна€ сила которых близка к нулю, коэффициенты активности единице, а значит активности равны концентраци€м. ¬ противном случае ионное произведение воды равно произведению активностей протонов и гидроксид-ионов:
воды = а (Ќ+) ∙ а (ќЌ-).
“ак, дл€ растворов с высокой концентрацией электролитов водородный показатель обозначаетс€ раЌ и равен отрицательному логарифму активной (эффективной) концентрации (активности) ионов водорода:
: р а Ќ = -lg([H+] Ј fH+) = -lg[H+] - lg fH+ = pH - lg fH+ , р а ќЌ = -lg([ќH-] Ј fHќ-) = -lg[ќH-] - lg fќH- = pќH - lg fќH-, где fH+ ― коэффициент активности протонов в данном растворе.
–ассмотрим несколько примеров расчета рЌ растворов.
ѕример 1. аково будет значение рЌ 0,001 нормального раствора ќЌ, ес-
ли допустить, что степень диссоциации щелочи в растворе 100%?
–ешение. √идроксид кали€ в водном растворе диссоциирует на ионы кали€
|
|
|
и гидроксила:
ќЌ → + + ќЌ-.
ѕоскольку диссоциаци€ растворенного гидроксида кали€ полна€, то концен-
траци€ гидроксил ионов ќЌ- равна 0,001 моль/л (так же как и ионов кали€). ”чи-
тыва€, что концентрации ионов водорода и гидроксила св€заны между собой
ионным произведением, равным [H+]Ј[OH-] = 10-14, найдем концентрацию ионов
водорода в этом растворе:
[H+] = 10-14 / [OH-] = 10-14 / 0,001 = 10-11 моль/л.
«на€ концентрацию ионов водорода, рассчитаем рЌ раствора: рЌ = -lg10-11 = 11.
ѕример 2. акова концентраци€ ионов гидроксила в растворе, рЌ которого
равен 5?
–ешение. ѕо величине рЌ определ€ем концентрацию ионов водорода Ќ+ в
растворе: так как рЌ = -lg [H+], то lg[H+] = -5 и [H+] = 10-5 моль/л. онцентра-
цию ионов ќЌ- рассчитываем исход€ из ионного произведени€ воды:
[OH-] = 10-14 / 10-5 = 10-9 моль/л.
ѕример 3. ћол€рные концентрации четырех двухкомпонентных растворов, содержащих фруктозу, H2SO4, HCl, CH3CO2H одинаковы и составл€ют 0,001ћ. ќсмотическое давление, создаваемое каким раствором будет наибольшее и наименьшее?

 –ешение. ќчевидно, что концентраци€ частиц наименьша€ в растворе фруктозы, потому что это органическое соединение, которое почти не диссоциирует в водных растворах. ”ксусна€ кислота Ц слабый электролит и диссоциирует неполностью. —тепень диссоциации
–ешение. ќчевидно, что концентраци€ частиц наименьша€ в растворе фруктозы, потому что это органическое соединение, которое почти не диссоциирует в водных растворах. ”ксусна€ кислота Ц слабый электролит и диссоциирует неполностью. —тепень диссоциации
α= =
—ерна€ и сол€на€ кислоты Ц сильные электролиты и диссоциируют полностью на ионы, но HCl распадаетс€ на две частицы, а H2SO4 Ц на три.
ѕолучаем следующие значени€ концентрации частиц в растворах:
‘руктоза Ц 0,0010 ћ
CH3CO2H Ц 0,0011 ћ
HCl - 0,0020 ћ
H2SO4 Ц 0,0030 ћ






