1. Резервуар ________________________________________________________________
2. Место, характер работы ____________________________________________________
3. Объект подготовлен к производству работ.
Ответственный за подготовку резервуара и коммуникаций
____________________________ _________________________
(должность, Ф.И.О.) (подпись)
(дата)
4. Перечень мер безопасности при подготовке резервуара к зачистке ____________________
5. Объект принят к производству работ.
Ответственный за проведение ремонта
____________________________ _________________________
(должность, Ф.И.О.) (подпись)
(дата)
6. Перечень мероприятий, обеспечивающих безопасность при проведении работ, режим работы в резервуаре ___________________________________________________
7. Защитные средства, приборы и приспособления, обеспечивающие безопасность работ внутри резервуара __________________________________________________
(перечислить защитные средства, приборы и приспособления, обеспечивающие безопасность работ)
8. Состав бригады и отметки о прохождении инструктажа
| Ф.И.О. | Должность | Подпись инструктируемого | Подпись проводящего инструктаж |
9. Наблюдение осуществляет____________________________________________
10. Периодичность анализа воздушной среды, результаты газового анализа
__________________________________ _______________________________
(дата) (подпись) (результаты анализа)
__________________________________ _______________________________
(дата) (подпись) (результаты анализа)
11. По окончании работ по зачистке и дегазации остаточная пожарная нагрузка в точках отбора проб составила _______________________________________________________
(указать места отбора проб и результаты анализа)
______________________________________
(подпись проводивших анализ)
12. Результаты газового анализа _______________________________________________
______________________________________
(подпись проводивших анализ)
Наряд-допуск выдал ________________________________________________________
(Ф.И.О.) (подпись)
Наряд закрыл_______________________________________________________________
(Ф.И.О.) (подпись)
Приложение 3
ХАРАКТЕРИСТИКА МОЮЩЕГО СРЕДСТВА «ТЕМП-300»
1. Моющее средство «Темп-300» - мелкокристаллический порошок светло-серого цвета, представляет собой смесь щелочных неорганических солей, поверхностно-активных веществ и полиэлектролита деэмульгатора с антистатическими свойствами.
2. Моющее средство «Темп-300» химически не окисляется, не полимеризуется. В воздушной среде и сточных водах токсичных соединений не образует; поверхностно-активные вещества, входящие в состав моющего средства, биологически разлагаемы, степень полного биораспада 95%. Специальных методов обезвреживания или уничтожения средства не требуется, т. к. оно относится к пожаровзрывобезопасным и неопасным (по токсикологической характеристике) продуктам.
3. Ориентировочно допустимый уровень (ОДУ) для водоемов 0,14+0,03 мг/дм3 в расчете на ПАВ или 2,2 мг/дм3 в расчете на товарную форму. ОДУ для сброса в аэротенки - 19,5 мг/дм3 в расчете на ПАВ или 300 мг/дм3 в расчете на товарную форму.
4. Для суммарного количественного анализа ПАВ в очищенной воде используется методика калориметрического определения с фосфорно-вольфрамовой кислотой (Лурье Ю. Ю. Аналитическая химия промышленных сточных вод. - М. Химия, 1984 - С. 358-360).
5. Специальные требования к личной гигиене и технике безопасности при приготовлении моющего раствора сводятся к защите глаз от прямого попадания порошка, пользованию рукавицами с защитными свойствами для щелочей Ш-20 и противопылевым респиратором.
6. Средство «Темп-300» должно храниться в многослойных бумажных или полиэтиленовых мешках весом 30 кг в сухих, крытых, проветриваемых помещениях в штабелях высотой не более 3 м с проходами между ними для циркуляции воздуха. Гарантийный срок хранения 12 месяцев с момента изготовления. По истечении гарантийного срока продукт анализируется на содержание массовой доли общей щелочности.
7. Общая щелочность препарата, %, не более 29, что соответствует в водных растворах с рабочей концентрацией показателю концентрации водородных ионов рН = 10,5¸11,0 по ГОСТ 2567.5-77.
8. Определение массовой доли общей щелочности. Навеску препарата массой 5 г помещают в химический стакан и растворяют в объеме 200 см3 горячей (60 °С) водой. Раствор охлаждают до комнатной температуры, переносят в мерную колбу, доводят до метки водой и тщательно перемешивают. Отбирают 100 см3 раствора и помещают в колбу для титрования вместимостью 250 см3, добавляют 1-2 капли метилового оранжевого (метиловый оранжевый - раствор с массовой долей 0,1 %, приготовленный по ГОСТ 4919.1-77), титруют раствором соляной кислоты (кислота соляная, ГОСТ 3118-77, х. ч., раствор с концентрацией HCl = 0,5 моль/дм3 или 0,5 Н) до изменения окраски из желтой в розово-оранжевую.
9. Массовую долю общей щелочности в пересчете на Na2O в процентах вычисляют по формуле
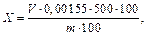
10. где V - объем раствора соляной кислоты концентрацией 0,5 моль/дм3, израсходованной на титрование, см3; 0,00155 - масса оксида натрия, соответствующая 1 см3 раствора соляной кислоты концентрацией 0,5 моль/дм3, г; m - масса навески, г.
11. Моющее средство «Темп-300» применяется в виде водных растворов концентрацией от 5 до 10 г/дм3 при температуре 50-80 °С.
Приложение 4






