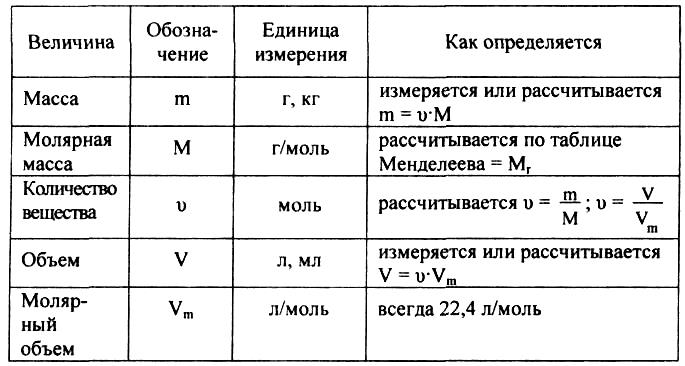
јтомно-молекул€рное учение определ€ет атом, как мельчайшую химически неделимую частицу. ј если это частица, то она должна иметь массу, котора€ очень мала. —овременные методы исследовани€ позвол€ют с большой точностью определ€ть эту величину.
 ѕример: m(H) = 1,674Ј 10-27кг
ѕример: m(H) = 1,674Ј 10-27кг
m(O) = 2,667 Ј 10-26 кг јбсолютные массы
m (C) = 1,993 Ј 10-26 кг

ѕредставленные величины очень неудобны дл€ проведени€ вычислений. ѕоэтому в химии чаще используют не абсолютные, а относительные атомные массы. ќтносительна€ атомна€ масса (јr) представл€ет собой отношение абсолютной массы атома к 1/12 массы атома углерода. — помощью формулы Ч это можно записать так 
1/12m(c) €вл€етс€ величиной сравнени€ и называетс€ 1 а.е.м.
1а.е.м. = 1/12Ј 1,993 Ј 10-26 кг = 1,661 Ј 10-27 кг
ѕосчитаем јr дл€ некоторых элементов.
јr(ќ) = 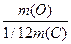 =
=  = 15,99 ~ 16
= 15,99 ~ 16
јr(H) =  =
=  = 1,0079 ~ 1
= 1,0079 ~ 1
—равнива€ относительные атомные массы кислорода и водорода с абсолютными, хорошо видны преимущества јr. ¬еличины јr намного проще. »х удобнее использовать в вычислени€х. √отовые величины јr приведены в таблице ћенделеева. »спользу€ јr элементов, можно проводить сравнени€ их масс.
n=  =
=  = 2,1
= 2,1
ƒанное вычисление показывает, что атом цинка весит в 2,1 раза больше, чем атом фосфора.
ќтносительна€ молекул€рна€ масса (Mr) равна сумме относительных атомных масс, вход€щих в нее атомов (безразмерна). ¬ычислим относительную молекул€рную массу воды. ¬ы знаете, что в состав молекулы воды вход€т два атома водорода и один атом кислорода. “огда ее относительна€ молекул€рна€ масса будет равна сумме произведений относительной атомной массы каждого химического элемента на число его атомов в молекуле воды:

вычислите относительные молекул€рные массы веществ.
Mr (Cu2O)= 143,0914
Mr (Na3PO4)= 163,9407
Mr (AlCl3)= 133,3405
Mr (Ba3N2)= 439,9944
Mr (KNO3)= 101,1032
Mr (Fe (OH)2)= 89,8597
Mr (Mg(NO3)2)= 148,3148
Mr (Al2(SO4)3)= 342,1509
оличество вещества (n) Ч физическа€ величина, характеризующа€ количество однотипных структурных единиц, содержащихс€ в веществе. ѕод структурными единицами понимаютс€ любые частицы, из которых состоит вещество (атомы, молекулы, ионы, электроны или любые другие частицы).
≈диницей измерени€ количества вещества (n) €вл€етс€ моль. ћоль Ц количество вещества, содержащее столько структурных элементарных единиц (молекул, атомов, ионов, электронов и т.д.), сколько содержитс€ атомов в 0,012 кг (12 г) = 1 моль изотопа углерода 12—.

„исло атомов NA в 0,012 кг (12 г) углерода, или в 1 моль, легко определить следующим образом:
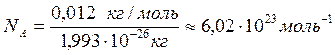 .
.
¬еличина NA называетс€ посто€нной јвогадро.
ѕри описании химических реакций, количество вещества €вл€етс€ более удобной величиной, чем масса, так как молекулы взаимодействуют независимо от их массы в количествах, кратных целым числам.
Ќапример, дл€ реакции горени€ водорода (2H2 + O2 → 2H2O) требуетс€ в два раза большее количество вещества водорода, чем кислорода. —оотношение между количествами реагирующих веществ непосредственно отражаетс€ коэффициентами в уравнени€х.
|
|
|
ѕример: в 1 моле хлорида кальци€ = содержит 6,022×1023 молекул (формульных единиц) - CaCl2.
1 моль (1 ћ) железа = 6 . 1023 атомов Fe
1 моль (1 ћ) ионов хлора Cl- = 6 . 1023 ионов Cl-.
1 моль (1 ћ) электронов е- = 6 . 1023 электронов е-.
ƒл€ вычислени€ количества вещества на основании его массы пользуютс€ пон€тием мол€рна€ масса:
ћол€рна€ масса (ћ) - это масса одного мол€ вещества (кг/моль, г/моль). ќтносительна€ молекул€рна€ масса и мол€рна€ масса вещества численно совпадают, но имеют разную размерность, например, дл€ воды ћr = 18 (относительна€ атомна€ и молекул€рна€ массы величины безразмерные), ћ = 18 г/моль. оличество вещества и мол€рна€ масса св€заны простым соотношением:

Ѕольшую роль в формировании химической атомистики сыграли основные стехиометрические законы, которые были сформулированы на рубеже XVII и XVIII столетий.
1. «ј ќЌ —ќ’–јЌ≈Ќ»я ћј——џ (ћ.¬. Ћомоносов,1748).
—умма масс продуктов реакции равна сумме масс исходных веществ. ¬ качестве дополнени€ к этому закону может служить закон сохранени€ массы элемента (1789, ј.Ћ. Ћавуазье) - масса химического элемента в результате реакции не измен€етс€. Ёти законы имеют дл€ современной химии определ€ющее значение, поскольку позвол€ют моделировать химические реакции уравнени€ми и выполн€ть на их основе количественные вычислени€.
2. «ј ќЌ ѕќ—“ќяЌ—“¬ј —ќ—“ј¬ј (∆. ѕруст,1799-1804).
»ндивидуальное химическое вещество молекул€рного строени€ имеет посто€нный качественный и количественный состав, не завис€щий от способа его получени€. —оединени€, подчин€ющиес€ закону посто€нства состава, называют дальтонидами. ƒальтонидами €вл€ютс€ все известные к насто€щему времени органические соединени€ (около 30 миллионов) и часть (около 100 тыс.) неорганических веществ. ¬ещества, имеющие немолекул€рное строение (бертолиды), не подчин€ютс€ данному закону и могут иметь переменный состав, завис€щий от способа получени€ образца. ним относ€тс€ большинство (около 500 тыс.) неорганических веществ.
3. «ј ќЌ Ё ¬»¬јЋ≈Ќ“ќ¬ (». –ихтер, ƒж. ƒальтон, 1792-1804).
аждое сложное вещество, независимо от способа его получени€, имеет посто€нный качественный и количественный состав. —ледовательно, химические вещества взаимодействуют друг с другом в строго определенных (эквивалентных) соотношени€х. ћассы реагирующих веществ пр€мо пропорциональны их эквивалентным массам.
 ,
,
где Ёј и Ё¬ - эквивалентные массы реагирующих веществ.
4. «ј ќЌ ј¬ќ√јƒ–ќ (ј. јвогадро,1811).
¬ равных объемах разных газов, измеренных в одинаковых услови€х (давление, температура), содержитс€ одинаковое число молекул. »з закона следует, что:
Ø ѕри нормальных услови€х (н.у., “ = 273 , р = 101,325 кѕа) один моль любого газа занимает одинаковый объем - мол€рный объем (Vm), равный 22,4 л/моль.

Ø ќтношение масс равных объемов разных газов, измеренных в одинаковых услови€х (относительна€ плотность газа по газу), равна отношению их молекул€рных (мол€рных) масс.


„аще всего определ€ют относительную плотность по водороду или воздуху. —оответственно,
 ,
,
где 29 - средн€€, точнее средневзвешенна€, молекул€рна€ масса воздуха.
Ø ќбъемы реагирующих газов относ€тс€ друг к другу и к объемам газообразных продуктов реакции как простые целые числа (закон объемных отношений √ей-Ћюссака).
|
|
|
«адача
—колько граммов газообразного хлора нужно потратить и сколько граммов жидкого хлорида фосфора(III) получитьс€ если в реакции использовано 1,45 граммов фосфора?
–4 (тв.) + Cl2 (г.) = PCl3 (ж.)
–ешение: 1. Ќеобходимо убедитьс€, что уравнение находитьс€ в равновесии, т.е. необходимо проставить стехиометрические коэффициенты: –4 (тв.) + 6Cl2 (г.) = 4PCl3 (ж.). Ќа 1 моль –4 € могу потратить 6 моль Cl2, чтобы получить 4 мол€ PCl3
2. ” нас есть масса –4 в реакции, следовательно, можно узнать сколько молей фосфора использовано. ѕо “.ћ. узнаем атомную массу фосфора ~ 31, это говорит, что 1 моль фосфора будет иметь массу 31 г (мол€рна€ масса), а атомна€ масса –4 будет 124 г. Ќайдем сколько молей в 1,45 г фосфора:
1,45 г Ц х моль х=0,0117 моль
124 г Ц 1 моль
3. “еперь узнаем сколько молей хлора нужно вз€ть дл€ использовани€ 0,0117 молей фосфора. ѕо равновесной реакции мы видим, что на 1 моль фосфора нужно вз€ть 6 молей хлора, следовательно, хлора нужно вз€ть в 6 раз больше. —читаем:
0,0117 х 6 = 0,07 молей хлора.
4. “еперь можем посчитать сколько граммов хлора нужно потратить:
0,07 молей х 70,906 г (в 1 моле Cl2) = 4,963 г Cl2
5. “еперь найдем сколько граммов жидкого хлорида фосфора(III) получитьс€. ћожно воспользоватьс€ двум€ разными решени€ми:
5.1. «акон сохранени€ массы 1,45г –4 (тв.) + 4,963 г. Cl2 (г.) = 6,413 г. PCl3 (ж.)
5.2. ј можно воспользоватьс€ способом как мы находили массу необходимого фосфора.
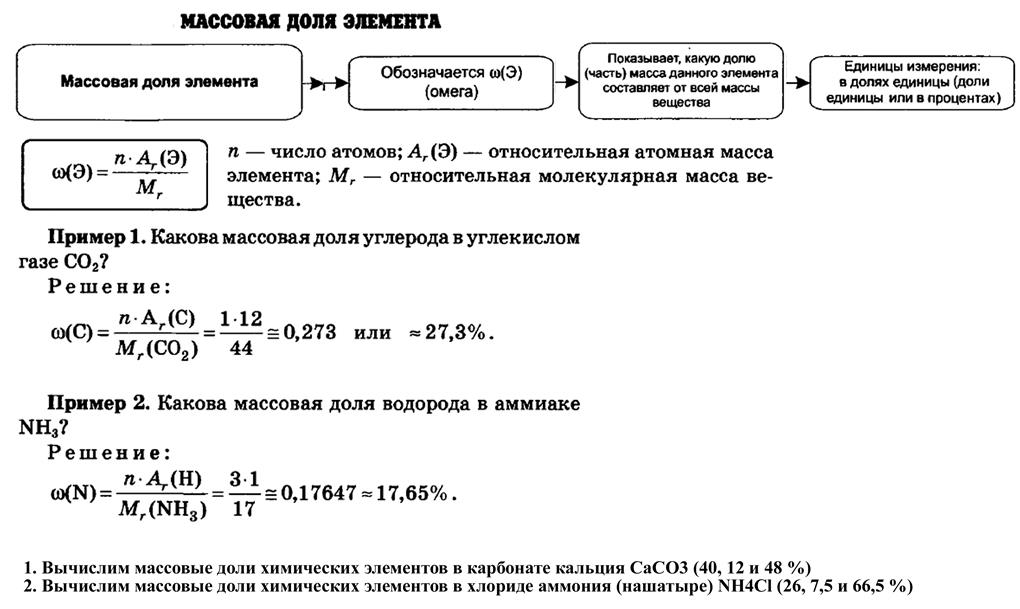
ѕримеры:
”словие
ќпределите массовую долю кристаллизационной воды в дигидрате хлорида бари€ BaCl2 Х 2H2O
–ешение
ћол€рна€ масса BaCl2 Х 2H2O составл€ет:
ћ(BaCl2 Х 2H2O) = 137+ 2 Х 35,5 + 2 Х 18 =244 г/моль
»з формулы BaCl2 Х 2H2O следует, что 1 моль дигидрата хлорида бари€ содержит 2 моль Ќ2ќ.
ќпредел€ем массу воды, содержащейс€ в BaCl2 Х 2H2O: m(H2O) = 2 Х 18 = 36 г.
Ќаходим массовую долю кристаллизационной воды в дигидрате хлорида бари€
BaCl2 Х 2H2O. ω(H2O) = m(H2O)/ m(BaCl2 Х 2H2O) = 36/244 = 0,1475 = 14,75%.

ѕример самосто€тельно
1. ’имическое соединение содержит по массе 17,56% натри€, 39,69% хрома и 42,75% кислорода. ќпределите простейшую формулу соединени€. (Na2Cr2O7).
2. Ёлементный состав вещества следующий: массова€ дол€ элемента железа 0,7241 (или 72,41%), массова€ дол€ кислорода 0,2759 (или 27,59%). ¬ыведите химическую формулу. (Fe3O4)
ѕример (разбор). ”становите молекул€рную формулу вещества, если массова€ дол€ углерода в нем составл€ет 26,67%, водорода Ц 2,22%, кислорода Ц 71,11%. ќтносительна€ молекул€рна€ масса этого вещества равна 90.
–ешение
1. ƒл€ решени€ задачи используем формулы: w =  ; n = ; n =  ; x: y: z = n(C): n(H): n(O).
2. Ќаходим химические количества элементов, вход€щих в состав вещества, прин€в, что m(CxHyOz) = 100 г.
m(C) = w(C) Ј m(CxHyOz) = 0,2667 Ј 100 г = 26,67 г.
m(H) = w(H) Ј m(CxHyOz) = 0,0222 Ј 100 г = 2,22 г.
m(O) = w(O) Ј m(CxHyOz) = 0,7111 Ј 100 г = 71,11 г.
n(C) = ; x: y: z = n(C): n(H): n(O).
2. Ќаходим химические количества элементов, вход€щих в состав вещества, прин€в, что m(CxHyOz) = 100 г.
m(C) = w(C) Ј m(CxHyOz) = 0,2667 Ј 100 г = 26,67 г.
m(H) = w(H) Ј m(CxHyOz) = 0,0222 Ј 100 г = 2,22 г.
m(O) = w(O) Ј m(CxHyOz) = 0,7111 Ј 100 г = 71,11 г.
n(C) =  = =  = 2,22 моль.; n(H) = = 2,22 моль.; n(H) =  = =  = 2,22 моль.; n(O) = = 2,22 моль.; n(O) = 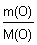 = =  = 4,44 моль.
3. ќпредел€ем эмпирическую формулу вещества:
n(C): n(H): n(O) = 2,22 моль: 2,22 моль: 4,44 моль.
x: y: z = 1: 1: 2.
Ёмпирическа€ формула вещества Ц CHO2.
4. ”станавливаем истинную молекул€рную формулу вещества:
Mr(CHO2) = Ar(C) + Ar(H) + 2Ar(O) = 12 + 1 + 2Ј16 = 45;
Mr(CHO2): Mr(CxHyOz) = 45: 90 = 1: 2.
»стинна€ молекул€рна€ формула вещества Ц C2H2O4.
ќтвет: молекул€рна€ формула вещества C2H2O4.
«адача. Ќайдите химическую формулу вещества, в состав которого входит 9 мас. ч. алюмини€ и 8 мас. ч. кислорода.
–ешение:
Ќаходим отношение числа атомов: = 4,44 моль.
3. ќпредел€ем эмпирическую формулу вещества:
n(C): n(H): n(O) = 2,22 моль: 2,22 моль: 4,44 моль.
x: y: z = 1: 1: 2.
Ёмпирическа€ формула вещества Ц CHO2.
4. ”станавливаем истинную молекул€рную формулу вещества:
Mr(CHO2) = Ar(C) + Ar(H) + 2Ar(O) = 12 + 1 + 2Ј16 = 45;
Mr(CHO2): Mr(CxHyOz) = 45: 90 = 1: 2.
»стинна€ молекул€рна€ формула вещества Ц C2H2O4.
ќтвет: молекул€рна€ формула вещества C2H2O4.
«адача. Ќайдите химическую формулу вещества, в состав которого входит 9 мас. ч. алюмини€ и 8 мас. ч. кислорода.
–ешение:
Ќаходим отношение числа атомов:
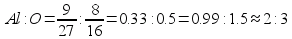 ќтвет: ’имическа€ формула данного вещества:
ќтвет: ’имическа€ формула данного вещества:  .
ќтносительна€ плотность газа ’ по газу ” Ч Dпо”(’).
„асто в задачах прос€т определить формулу вещества (газа) в зависимости от ќтносительной плотности D Ч это величина, котора€ показывает, во сколько раз газ ’ т€желее газа ”.
≈Є рассчитывают как отношение мол€рных масс газов ’ и ”: Dпо”(’) = ћ(’) / ћ(”) „асто дл€ расчетов используют относительные плотности газов по водороду и по воздуху. ќтносительна€ плотность газа ’ по водороду: Dпо H2 = M(газа ’) / M(H2) = M(газа ’) / 2 ¬оздух Ч это смесь газов, поэтому дл€ него можно рассчитать только среднюю мол€рную массу. ≈Є величина прин€та за 29 г/моль (исход€ из примерного усреднЄнного состава). ѕоэтому: Dпо возд. = ћ(газа ’) / 29
ѕример: ќпределить формулу вещества, если оно содержит 84,21% — и 15,79% Ќ и имеет относительную плотность по воздуху, равную 3,93. ѕусть масса вещества равна 100 г. “огда масса — будет равна 84,21 г, а масса Ќ Ч 15,79 г.
1. ЌайдЄм количество вещества каждого атома: ν(C) = m / M = 84,21 / 12 = 7,0175 моль, ν(H) = 15,79 / 1 = 15,79 моль.
2.ќпредел€ем мольное соотношение атомов — и Ќ: —: Ќ = 7,0175: 15,79 (поделим оба числа на меньшее) = 1: 2,25 (будем домножать на 1, 2,3,4 и т.п. пока после зап€той не по€витс€ 0 или 9. ¬ данной задаче нужно домножить на 4) = 4: 9. “аким образом, простейша€ формула Ч —4Ќ9.
3. ѕо относительной плотности рассчитаем мол€рную массу: ћ = D(возд.) Х 29 = 114 г/моль. ћол€рна€ масса, соответствующа€ простейшей формуле —4Ќ9 Ч 57 г/моль, это в 2 раза меньше истинно мол€рной массы. «начит, истинна€ формула Ч —8Ќ18. .
ќтносительна€ плотность газа ’ по газу ” Ч Dпо”(’).
„асто в задачах прос€т определить формулу вещества (газа) в зависимости от ќтносительной плотности D Ч это величина, котора€ показывает, во сколько раз газ ’ т€желее газа ”.
≈Є рассчитывают как отношение мол€рных масс газов ’ и ”: Dпо”(’) = ћ(’) / ћ(”) „асто дл€ расчетов используют относительные плотности газов по водороду и по воздуху. ќтносительна€ плотность газа ’ по водороду: Dпо H2 = M(газа ’) / M(H2) = M(газа ’) / 2 ¬оздух Ч это смесь газов, поэтому дл€ него можно рассчитать только среднюю мол€рную массу. ≈Є величина прин€та за 29 г/моль (исход€ из примерного усреднЄнного состава). ѕоэтому: Dпо возд. = ћ(газа ’) / 29
ѕример: ќпределить формулу вещества, если оно содержит 84,21% — и 15,79% Ќ и имеет относительную плотность по воздуху, равную 3,93. ѕусть масса вещества равна 100 г. “огда масса — будет равна 84,21 г, а масса Ќ Ч 15,79 г.
1. ЌайдЄм количество вещества каждого атома: ν(C) = m / M = 84,21 / 12 = 7,0175 моль, ν(H) = 15,79 / 1 = 15,79 моль.
2.ќпредел€ем мольное соотношение атомов — и Ќ: —: Ќ = 7,0175: 15,79 (поделим оба числа на меньшее) = 1: 2,25 (будем домножать на 1, 2,3,4 и т.п. пока после зап€той не по€витс€ 0 или 9. ¬ данной задаче нужно домножить на 4) = 4: 9. “аким образом, простейша€ формула Ч —4Ќ9.
3. ѕо относительной плотности рассчитаем мол€рную массу: ћ = D(возд.) Х 29 = 114 г/моль. ћол€рна€ масса, соответствующа€ простейшей формуле —4Ќ9 Ч 57 г/моль, это в 2 раза меньше истинно мол€рной массы. «начит, истинна€ формула Ч —8Ќ18.
| |
|
|
|






