’ими€
ћетодические указани€, контрольные задани€
и вопросы дл€ подготовки к экзаменам
дл€ студентов Ц заочников
© ибенко ¬. ƒ. 2002
©»здательство »ж√“”, 2002
»жевск 2002
”ƒ 54
—оставитель ¬. ƒ. ибенко
’ими€
ћетодические указани€, контрольные задани€ и вопросы дл€ подготовки к экзаменам дл€ студентов Ц заочников. Ц »жевск: »ж√“”, 2002 Ц 37 с.
¬ работе представлены методические указани€ по изучению дисциплины ''’ими€'', варианты контрольных заданий по основным разделам курса и вопросы, выносимые на экзамен с типовыми задачами. ѕриведены основные справочные данные, необходимые дл€ решени€ контрольных заданий и список литературы.
ѕредназначена дл€ студентов Ц заочников 1 курса всех специальностей, изучающих курс химии в »жевском государственном техническом университете.
–екомендована к изданию на заседании кафедры
''’ими€ и химическа€ технологи€''
»ж√“”, 30 ма€ 2002 г.
ќбщие методические указани€
’ими€, €вл€€сь одной из фундаментальных естественно - научных дисциплин, изучает материальный мир, законы его развити€, химическую форму движени€ материи. «нание химии необходимо дл€ плодотворной творческой де€тельности инженера любой специальности.
ќсновным видом учебных зан€тий студентов Ц заочников €вл€етс€ самосто€тельна€ работа над учебным материалом. ¬ курсе она включает изучение дисциплины по учебникам и учебным пособи€м, выполнение контрольных заданий, выполнение лабораторных работ, индивидуальные консультации, посещение лекций, сдачу зачетов по лабораторному практикуму и сдачу экзамена по всему курсу.
онтрольные работы не должны быть самоцелью; они €вл€ютс€ формой методической помощи студентам при изучении курса. –ешение задач Ц один из лучших методов прочного усвоени€, проверки и закреплени€ теоретического материала. выполнению контрольного задани€ можно приступать только тогда, когда по учебнику и учебным пособи€м будет усвоена соответствующа€ тема курса и тщательно разобраны решени€ примеров типовых задач по данной теме.
ѕри первом чтении темы курса по учебнику не задерживайтесь на математических выводах, составлении уравнений реакций; старайтесь получить общее представление по изучаемым вопросам, а также отмечайте трудные или не€сные места.
ѕри повторном изучении темы усвойте все теоретические положени€, математические зависимости и их выводы, а также принципы составлени€ уравнений реакций.
¬никайте в сущность того или иного вопроса, а не пытайтесь запомнить отдельные факты и €влени€. »зучение любого вопроса на уровне сущности, а не на уровне отдельных €влений способствует более глубокому и прочному усвоению материала.
„тобы лучше запомнить и усвоить изучаемый материал, надо об€зательно иметь рабочую тетрадь и заносить в нее формулировки законов и основных пон€тий химии, новые незнакомые термины и названи€, формулы и уравнени€ реакций, математические зависимости и их выводы. ¬о всех случа€х, когда материал поддаетс€ систематизации, составл€йте графики, схемы, диаграммы, таблицы. ќни очень облегчают запоминание и уменьшают объем конспектируемого материала. ѕока тот или иной раздел не усвоен, переходить к изучению новых разделов не следует. раткий конспект курса будет полезен при повторении материала в период подготовки к экзамену.
|
|
|
онтрольна€ работа должна быть аккуратно оформлена. –ешение задач и ответы на теоретические вопросы должны быть коротко, но четко обоснованы. ѕри решении задач нужно приводить весь ход решени€ и математические преобразовани€. „исленные значени€ рассчитанных величин приводить с разумной точностью и указывать их размерность. ƒл€ замечаний рецензента надо оставл€ть широкие пол€; писать четко и €сно; номера и услови€ задач переписывать в том пор€дке, в каком они указаны в задании. ¬ конце работы следует привести список использованной литературы с указанием года издани€. –аботы должны быть датированы, подписаны студентом и представлены на рецензирование.
≈сли контрольна€ работа не зачтена, еЄ нужно выполнить повторно в соответствии с указани€ми рецензента. »справлени€ следует выполн€ть в конце тетради, а не в рецензированном тексте. онтрольна€ работа, выполненна€ не по своему варианту, преподавателем не рецензируетс€ и не засчитываетс€ как сделанна€.
сдаче экзамена допускаютс€ студенты, которые выполнили контрольные задани€ и сдали зачет по лабораторному практикуму. Ёкзаменатору студенты предъ€вл€ют зачетную книжку и зачтенные контрольные работы.
онтрольное задание є1
“ема: Ђ—троение атомаї
1. —оставьте электронные формулы дл€ указанных элементов.
2. какому семейству(s, p, d, f) они относ€тс€?
3. ѕокажите распределение электронов по энергетическим €чейкам.
4. ќпределите валентные электроны.
5. ќпределите валентность в невозбужденном и возбужденном состо€ни€х.
| єва-риа-нта | ѕор€дковый номер элемента | єва-риа-нта | ѕор€дковый номер элемента | єва-риа-нта | ѕор€дковый номер элемента |
| 19,74 40,76 15,24 30,53 22,46 20,83 25,34 16,48 32,17 42,57 | 29,52 33,43 82,21 12,44 28,72 7,77 5,89 37,45 27,88 26,79 | 23,51 8,75 49,73 31,80 47,50 9,81 39,85 14,41 35,56 13,84 |
онтрольное задание є 2
“ема: Ђ’имическа€ св€зьї
1. ¬ывести электронно-точечные формулы молекул и ионов.
2. ќпределить тип гибридизации.
3. ”становить геометрическую форму молекулы или иона.
4. ќпределить характер св€зей (ионна€, ковалентна€), наличие и число π-св€зей.
| єва-риа-нта | ‘ормула молекул или ионов | єва-риа-нта | ‘ормула молекул или ионов | єва-риа-нта | ‘ормула молекул или ионов |
| H2O, SF6 NH3, SnCl2 PH3, TeCl4 SbH3, BF4‾, SnCl4, NO3‾ SOCl2, SiCl4 XeF4, ClF3 ClF5, AsO43Ц PCl6ˉ, SeF4 (CH3)2S, CO2 | NH2Cl, OF2 PCI3, NO2‾ COCI2, NH4+ POCI3, COF2 BCI3, CO32Ц IF7, SeO42Ц CCI4, PH4+ SCI4, H2S PCI5, HCIO4 PF3, BaCI2 | CS2, CIO2‾ H2Te, IF3 CF4, SiO44Ц [Al(OH4)]‾, NF3 SOCI2, H3O+ PBr3, SOF2 POF3, CBr4 BBr3, SOCI4 SF4, PCI5 COBr2, PCI3 |
онтрольное заданиеє 3
“ема: ЂЁнергетика химических процессовї
1. ¬ычислить стандартную теплоту образовани€ бензола —6Ќ6(ж), если известны теплоты сгорани€ водорода, углерода и бензола.
2. ќпределить стандартную теплоту образовани€ этилового спирта, если теплоты сгорани€ углерода, водорода и этилового спирта соответственно равны: Ц393,51; Ц285,84;Ц1366,91 кƒж/моль.
|
|
|
3. ¬ычислите изменение энергии √иббса при 25∞ и 727∞— дл€ реакции:
—(графит) + H2O(г) = H2(г) + CO(г) (¬ли€нием температуры на ΔЌ∞298 и ΔS∞298 пренебречь)
а) при каком из указанных температурных условий принципиально возможно протекание реакции?
б) какой фактор Ц энтальпийный или энтропийный Ц определ€ет возможность протекани€ этой реакции?
4. ¬ычислите ΔЌ∞298 хлорида аммони€, если дл€ реакции:
NH3(г) + Ќ—1(г) = NЌ4—l(т), ΔЌ∞298 = Ц176,93кƒж.
5. ќкисление аммиака протекает по уравнению:
4NЌ3(г) + 3O2(г) = 2N2(г) + бЌ2O(ж); ΔЌ∞298= Ц1528кƒж.
ќпределите стандартную теплоту образовани€ NЌ3 и NH4ќЌ, если теплота растворени€ NH3 в воде равна Ц34,65кƒж.
6. ¬осстановление диоксида свинца водородом протекает по уравнению:
–№O2(т) + Ќ2(г) = –bќ(желтый) + H2O, ΔЌ∞298= Ц182,8кƒж. ќпределите стандартную теплоту образовани€ –bќ2(т).
7. ¬ычислив ΔG∞298 системы –bO2(т) + –b(т) = 2–bќ(жЄлтый) на основании ΔЌ∞298 и S∞298 реагирующих веществ, определите, возможна ли эта реакци€.
8. ”кажите, какие из приведЄнных реакций протекают самопроизвольно и €вл€ютс€ экзотермическими:
а) 2Ќ2O2(ж) = 2Ќ2O(ж) + O2(г)
б) 3Ќ2(г) + N2(г) = 2NЌ3(г)
в) N2O4(г) = 2NO2(г)
9. –еакци€ восстановлени€ Fе2O3 водородом протекает по уравнению:
Fе2O3(т) + 3Ќ2(г) = 2Fe(т) + 3Ќ2O(г) ¬озможна ли эта реакци€ при стандартных услови€х. ѕри какой температуре начнЄтс€ восстановление Fе2O3?
10. ¬ычислите ΔЌ∞, ΔS∞, ΔG∞ реакции, протекающей по уравнению:
Fе2O3(т) + 3—(т) = 2Fе(т) + 3—ќ(г). ¬озможна ли реакци€ восстановлени€ Fе2O3 углеродом при температуре 500 и 1000 ?
11. ѕри какой температуре наступит равновесие системы:
4Ќ—1(г) + O2(г) = 2Ќ2O(г) + 2—12(г);
’лор или кислород в этой системе €вл€ютс€ наиболее сильным окислителем и при каких температурах?
12. ¬ычислите, сколько теплоты выделитс€ при сгорании 165л (н.у.) ацетилена —2H2, если продуктами сгорани€ €вл€ютс€ диоксид углерода и пары воды.
13. ќпределите, при какой температуре начнЄтс€ реакци€ восстановлени€Fе3O4, протекающа€ по уравнению:
Fе3O4(т) + —ќ(г) = «Fеќ(т) + —ќ2(г).
14. –еакци€ горени€ этилового спирта выражаетс€ термохимическим уравнением —2Ќ5OH(ж) + O2(г) = 2—O2(г) + 3Ќ2O(ж), ΔЌ=?. ¬ычислить тепловой эффект реакции, если известно, что мольна€ теплота парообразовани€ —2Ќ5OЌ(ж) равна +42,36кƒж и известны теплоты образовани€ —2Ќ5OЌ(г); —ќ2(г); Ќ2O(ж).
15. акие из карбонатов: ¬е—ќ3, —а—ќ3 или ¬а—ќ3 Ц можно получить по реакции взаимодействи€ соответствующих оксидов с —ќ2? ака€ реакци€ идЄт более энергично? ¬ывод сделайте, вычислив ΔG∞298 реакций.
16. ¬ычислите изменение энтропии дл€ реакций, протекающих по уравнени€м:
2—Ќ4(г) = —2Ќ2(г) + 3Ќ2(г)
N2(г) + 3Ќ2(г) = 2NH3(г)
—(графит) + O2(г) = —ќ2(г)
ѕочему в этих реакци€х ΔS∞298>0, <0, = 0?
17. акой из оксидов: —аќ, јl2ќ3 или Znќ может быть восстановлен водородом до свободного металла? ќтвет подтвердить расчЄтом ΔG∞298 процесса.
18. ƒл€ каких оксидов: Rb2ќ, —uќ, ћnќ или –bќ принципиально осуществима реакци€ восстановлени€ водородом в стандартных услови€х. ќтвет подтвердите расчЄтом ΔG∞298процесса.
19. ¬ычислите, сколько теплоты выделитс€ при гашении 100 г извести при стандартных услови€х.
20. –ассчитать ΔG∞298 следующих реакций и установить в каком направлении они могут протекать самопроизвольно в стандартных услови€х:
а) 2ZnO(т) + —(графит) = 2Zn(т) + —ќ2(г)
б) Znќ(т) + Ќ2(г) = Zn(т) + Ќ2ќ(г)
в) ZnO(т) + ћg(т) = Zn(т) + ћgO(т)
21. –ассчитать ΔG∞298 реакции ћgќ(т) + —ќ2(г) = ћg—ќ3(т) и установить, в каком направлении она может протекать самопроизвольно в стандартных услови€х. ”становить температуру, при которой ћgќ и ћg—ќ3 наход€тс€ в равновесии с —O2.
22. ¬ычислить ΔG∞298 реакции Fе3O4(т) + 4Ќ2(г) = 3Fе(т) + 4Ќ2O(г) и установить, в каком направлении она может протекать самопроизвольно в стандартных услови€х. ќпределить температуру при которой она начнЄт протекать.
|
|
|
23. ¬ычислите ΔG∞298 реакции Fеќ(т) + Ќ2(г) = Fе(т) + Ќ2ќ(г) и установите, в каком направлении она может протекать самопроизвольно в стандартных услови€х. ќпределите температуру при которой она начнЄт протекать.
24. ¬ычислите ΔG∞298 реакции —аќ(т) + —ќ2(г) = —а—ќ3(т) и установите, в каком направлении она может протекать самопроизвольно в стандартных услови€х. ќпределите температуру, при которой —аќ и —а—ќ3 наход€тс€ в равновесии с —ќ2.
|
—а—N2(т) + «Ќ2ќ(г) = —а—ќ3(т) + 2NЌ3(г)
—а(т) + —(графит) + 3/2ќ2 = —а—ќ3(т)
1/2N2(г) + 3/2Ќ2(г) = NЌ3(г)
Ќ2(г) + 1/2ќ2(г) = Ќ2ќ(г)
26. »сход€ из величины ΔG∞298 соединений, участвующих в реакции, определите возможно ли самопроизвольное протекание реакции при стандартных услови€х:
јl2ќ3(т) + 3Sќ3(г) = ј12(Sќ4)3(т) ¬ычислить температуру равновеси€ дл€ этой реакции.
27. ¬ычислить теплотворную способность в кƒж/кг и ккал/кг угл€, содержащего 10% негорючих примесей.
28. ¬ычислить тепловой эффект реакции
2—2Ќ2(г) + 5O2(г) = 4—O2(г) + 2Ќ2O(г). —колько теплоты выделитс€ от сгорани€ 1 л ацетилена?
29 ¬озможно ли восстановление диоксида титана до свободного металла по схеме:
“iO2(т) + 2—(графит) = “i(т) + 2—ќ(г) при температурах 298 и 2500 ?
30 ¬ычислите при какой температуре становитс€ возможным протекание реакции:
–—l5(г) = –—13(г) + —12(г). ћожет ли она протекать самопроизвольно в стандартных услови€х?
онтрольное задание є 4
“ема: Ђ’имическа€ кинетика и равновесиеї
1. ’имическое равновесие реакции —ќ—l2(г) <=> —ќ(г) + —l2(г) установилось при концентраци€х реагирующих веществ (моль/л): 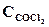 = 10; ——O = 2;
= 10; ——O = 2; 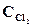 = 4. ¬ равновесную систему добавили хлор в количестве 4моль/л. ќпределить новые равновесные концентрации реагирующих веществ после смещени€ равновеси€.
= 4. ¬ равновесную систему добавили хлор в количестве 4моль/л. ќпределить новые равновесные концентрации реагирующих веществ после смещени€ равновеси€.
2. ¬ каком направлении сместитс€ равновесие реакции
—Ќ4(г) + Ќ2ќ(г) <=> —ќ(г) + «Ќ2(г) при уменьшении объЄма в 3 раза?
3. ¬ каком направлении сместитс€ равновесие реакции
2—ќ + 2Ќ2 <=> —Ќ4 + —ќ2, если концентрации всех реагирующих веществ уменьшить в 3 раза?
4. –еакци€ протекает по уравнению 4Ќ—1 + ќ2 <=> 2Ќ2ќ + 2—l2. ¬ каком направлении сместитс€ химическое равновесие, если концентрацию всех реагирующих веществ увеличить в 2 раза?
5. ќпределить равновесные концентрации водорода и йода в реакции Ќ2 + J2 <=> 2HJ, если их начальные концентрации составл€ли по 0,02 моль/л, а равновесна€ концентраци€ HJ равна 0,03 моль/л. ¬ычислить константу равновеси€.
6. онстанта равновеси€ реакции: N2 + «Ќ2 <=> 2NЌ3, р = 0,1 при 673 . –авновесные концентрации (моль/л): 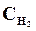 = 0,6;
= 0,6; 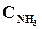 = 0,18. ¬ычислить начальную и равновесную концентрации азота.
= 0,18. ¬ычислить начальную и равновесную концентрации азота.
7. ѕри 393 реакци€ заканчиваетс€ за 18мин. „ерез сколько времени эта реакци€ закончитс€ при 453 , если температурный коэффициент скорости реакции равен «?
8. –еакци€ протекает по уравнению
Nа2S203 + Ќ2S04 = Nа2S04 + Ќ2S03 + S. ак изменитс€ скорость реакции после разбавлени€ реагирующей смеси в 4 раза?
9. ¬ начальный момент протекани€ реакции N2 + «Ќ2 <=> 2NЌ3 концентрации были равны (моль/л): 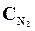 = 1,5;
= 1,5; 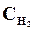 = 2,5;
= 2,5; 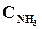 = 0. аковы концентрации азота и водорода тогда, когда концентраци€ аммиака 0,5моль/л?
= 0. аковы концентрации азота и водорода тогда, когда концентраци€ аммиака 0,5моль/л?
10. ¬ реакции N2 + «Ќ2 <=> 2NЌ3 равновесие установилось при следующих концентраци€х реагирующих веществ (моль/л): —N2 = 2,5; —H2 = 1,8; —NH3 = 3,6. –ассчитайте константу равновеси€ этой реакции и исходные концентрации азота и водорода.
|
|
|
11. Ќачальные концентрации веществ в реакции
—ќ(г) + Ќ2ќ(г) <=> —ќ2(г) + Ќ2(г) были равны (моль/л): —со = 0,5; —H2O =0,6; —CO2 = 0,4; —H2 = 0,2. ¬ычислить концентрации всех участвующих веществ в реакции после того, как прореагировало 60% Ќ2ќ.
12. –еакци€ идЄт по уравнению 4NЌ3 + 5ќ2 <=> 4Nќ + 6Ќ2ќ. ак изменитс€ скорость реакции, если увеличить давление в 2 раза?
13. ќпределите, как изменитс€ скорость пр€мой реакции
2Sќ2 + ќ2 <=> 2Sќ3, если общее давление в системе увеличить в 4 раза?
14. Ќапишите выражение дл€ константы равновеси€ обратимой реакции Fе—13(р) + « —NS(р) <=> Fе(—NS)3(р) + « —1(р). ¬ каком направлении будет смещатьс€ равновесие при добавлении к системе соответственно KCNS; Fе—13; —1 и как это отразитс€ на окраске системы? Fе(—NS)3 имеет интенсивно красную окраску.
15. ¬ смеси Nќ2 (бурого цвета) и N2O4 (бесцветен) протекает обратима€ реакци€: 2Nќ2 <=> N2O4, ∆Ќ= Ц54,3кƒж. ак будет вли€ть изменение температуры на состо€ние равновеси€ системы? ак это скажетс€ на изменение окраски смеси?
16. ¬ каком направлении произойдет смещение равновеси€ системы N2 + «Ќ2 <=> 2NЌ3, ∆Ќ= Ц92кƒж при понижении температуры ак объ€снить, что на практике синтез аммиака ведут при повышенной температуре (не ниже 400 Ц500∞—).
17. »сходные концентрации окиси азота и хлора в системе
2Nќ(г)+—l2(г)<=>2Nќ—1(г) составл€ют соответственно 0,5 моль/л и 0,2 моль/л. ¬ычислить константу равновеси€, если к моменту наступлени€ равновеси€ прореагировало 20% окиси азота.
18. ¬о сколько раз следует увеличить давление, чтобы скорость образовани€ Nќ2 по реакции 2NO(г)+ќ2<=>2Nќ2(г) возросла в 1000 раз?
19 ¬о сколько раз изменитс€ скорость пр€мой и обратной реакции в системе 2Sќ2(г) + ќ2(г) <=> 2Sќ3(г), если объЄм газовой смеси уменьшить в 3 раза. ¬ какую сторону сместитс€ равновесие системы.
20. онстанта равновеси€ гомогенной системы
—ќ(г) + Ќ2ќ(г) <=> —ќ2(г) + Ќ2(г), при 850∞— равна 1. ¬ычислить концентрации всех веществ при равновесии, если исходные концентрации —ќ и Ќ2ќ соответственно равны 3моль/л и 2моль/л.
21. –еакци€ идЄт по уравнению Ќ2(г) + J2(г)= 2HJ(г). онстанта скорости этой реакции при 508∞— равна 0,16 »сходные концентрации реагирующих веществ Ќ2 и J2 были соответственно 0,04моль/л и 0,05 моль/л. ¬ычислить начальную скорость реакции и скорость еЄ, когда концентраци€ H2 стала равной 0,03 моль/л.
22. Ќапишите выражение дл€ константы равновеси€ гетерогенной системы —O2(г) + C(т) <=> 2—ќ(г). ак изменитс€ скорость пр€мой реакции, если концентрацию —ќ2 уменьшить в 4 раза. ак следует изменить давление, чтобы повысить выход —ќ.
23. Ќапишите выражение дл€ константы равновеси€ гетерогенной системы —(т) + Ќ2ќ(г) <=> —ќ(г) + Ќ2(г). ак следует изменить концентрацию и давление, чтобы сместить равновесие в сторону обратной реакции.
24. онстанта равновеси€ реакции Fеќ(к) + —ќ(г) <=> Fе(к) + —ќ2(г) при некоторой температуре равна 0,5. Ќайти равновесные концентрации —ќ и —ќ2, если начальные концентрации этих веществ соответственно равны 0,05моль/л и 0.01моль/л.
25. Ќапишите выражени€ дл€ констант равновеси€ следующих реакций: 2Ќ2(г) + ќ2(г) <=> 2Ќ2ќ(г), ∆Ќ = Ц483,6кƒж
—(графит) + Ќ2ќ(г) <=> —ќ(г) + Ќ2(г), ∆Ќ = 131,3кƒж.
ак повли€ет на равновесие этих реакций
а) повышение температуры;
б) повышение давлени€.
26. ѕри некоторой температуре в реакции 2Sќ2 + ќ2 <=> 2Sќ3 установилось равновесие при следующих концентраци€х веществ (моль/л): 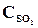 = 0,04,
= 0,04, 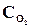 = 0,06,
= 0,06, 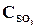 = 0,02. ¬ычислить константу равновеси€ этой реакции и исходные концентрации Sќ3 и ќ2.
= 0,02. ¬ычислить константу равновеси€ этой реакции и исходные концентрации Sќ3 и ќ2.
27. Ќапишите выражение константы равновеси€ реакции:
CO2(г) + —(графит) <=> 2—ќ(г), ∆Ќ = 172,5кƒж. ¬ каком направлении произойдет смещение равновеси€ этой реакции:
а) при повышении давлени€;
б) при повышении температуры;
в) при повышении концентрации —ќ.
28. ƒл€ реакции: 2SO2 + O2 <=> SO3 начальные концентрации исходных веществ были равны 1,6 моль/л Ц SO2 и 1,2 моль/л Ц ќ2. ¬ычислите константу равновеси€ этой реакции, если равновесна€ концентраци€ SO3 равна 0,6 моль/л?
29. Ќапишите выражение константы равновеси€ реакции:
2H2(г) + —(графит) <=> —Ќ4(г), ∆Ќ= Ц74,9кƒж.
¬ каком направлении произойдет смещение равновеси€ этой реакции:
а) при добавлении Ќ2;
б) при повышении давлени€;
в) при охлаждении.
30. ¬ычислить константу равновеси€ реакции CO + Cl2 <=> COCl2 если известно, что начальные концентрации CO и Cl2 были равны соответственно 0,28моль/л и 0,09моль/л, а равновесна€ концентраци€ —ќ равна 0,2моль/л.
|
|
|
онтрольное задание є 5
“ема: Ђƒиссоциаци€ и реакции ионного обменаї
| є варианта | 1. Ќаписать уравнени€ диссоциации следующих электролитов: | 2. Ќаписать в молекул€рной и молекул€рно- ионной формах уравнени€: | 3. —оставить по два молекул€рных уравнени€ реакции к каждому молекул€рно-ионному уравнению: | |
| 1. | H2CO3 ЌS | –b(Nќ3)2 + J→ —аCl2 + Nа2—ќ3→ | CO32Ц+2H+=CO2+H2O H++OH ‾=H2O | |
| 2. | Zn(ќЌ)2 MgOHCl | ¬а—12+ 2—rќ4→ (NЌ4)2—ќ3+—а(Nќ3)2→ | Pb2++2J ‾=PbJ2 NH4++OH ‾=NH3+H2O | |
| 3. | H2C2O4 2Ќ–ќ4 | AgNO3+ Fе—13→ ¬а(ќЌ)2+ЌNO3→ | Ca2++CO32Ц=CaCќ3 Fe3++3OH‾=Fe(OH)3 | |
| 4. | Cr2(SO4)3 —иќЌ—1 | CuCl2+NaOH→ Ba(Nќ3)2+K2SO4→ | Fe2++S2Ц=FeS HCO3Ц+OH‾=H2O+CO32Ц | |
| 5. | ј1(ќЌ)3 KHCO3 | CuSO4+Na2S→ Pb(CH3COO)2+KCl→ | Cu2++2OH‾=Cu(OH)2 Ni2++S2Ц = NiS | |
| 6. | Ќ2Sќ3 ј1(OЌ)2—1 | KCN+HCl→ CaCl2+Na3PO4→ | H++NO2‾=HNO2 Zn2++CO32Ц=ZnCO3 | |
| 7. | Cr(OH)3 NЌ4ЌS | ZnSO4+NaOH→ MnCl2+K2S→ | 3Ca2++2PO43Ц=Ca3(PO4)2 NH4++OH‾-=NH4OH | |
| 8. | Ќ3–ќ4 ј1(Nќ3)3 | NaHCO3+NaOH→ Ca(NO3)2+K2SO3→ | CN ‾+H+=HCN Ba2++SO42Ц=BaSO4 | |
| 9. | Na2Ќ–ќ4 NiOЌ—1 | NH4OH+NHO3→ Pb(NO3)2+K2S→ | Cu2++S2Ц=CuS Zn2++2OH‾=Zn(OH)2 | |
| 10. | FeќЌSO4 (NЌ4)2Ќ–ќ4 | AlCl3+NaOH→ AgNO3+Na2CO3→ | 3Mg2++2PO43Ц=Mg3(PO4)2 Cr3++3OH‾-=Cr(OH)3 | |
| 11. | —rќЌCl2 3–ќ4 | Zn(OH)2+HCl→ FeCl3+Na2S→ | Pb2++SO42Ц=PbSO4 2H++S2Ц=H2S | |
| 12. | Fе2(SO4)3 Sn(OH)2 | H2CO3+NaOH→ Pb(NO3)2+Na3PO4→ | Fe2++2OH‾=Fe(OH)2 Ag++Cl‾=AgCl | |
| 13. | H2SiO3 CrOHSO4 | Ba(OH)2+Na2CO3→ AlCl3+Na2S→ | Pb2++2OH‾=Pb(OH)2 CuOH++OH‾=Cu(OH)2 | |
| NaH2PO4 Cd(OH)2 | CaCl2+H3PO4→ AgNO3+BaJ2→ | 2Al3++3S2Ц=Al2S3 Ba2++CO32Ц=BaCO3 | ||
| KH2PO4 CrOH(NO3)2 | Cd(NO3)2+Na2CO3→ BaJ2+Cr2(SO4)3→ | HSO3‾+OH‾=SO32Ц+H2O Mn2++S2Ц=MnS | ||
| AlOHCl2 Co(OH)2 | SnCl2+Na3PO4→ Pb(NO3)2+K2S→ | H++CH3COO‾=CH3COOH Ni2++2OH‾=Ni(OH)2 | ||
| Cr(OH)2NO3 Ni(OH)2 | CoSO4+NaOH→ CuOHCl+HCl→ | 3Zn2++2PO43Ц=Zn3(PO4)2 Co2++S2Ц=CoS | ||
| HNO2 CrOHCl2 | ZnOHNO3+HNO3→ Al2(SO4)3+NaOH→ | 3Ba2++2PO43Ц=Ba3(PO4)2 Cd2++S2Ц=CdS | ||
| Cr2(SO4)3 NaHCO3 | Ba(NO3)2+K2CrO4→ CuOHCl+NaOH→ | Ca2++SO32Ц=CaSO3 3Ag++PO43Ц=Ag3PO4 | ||
| AlOHSO4 Na3PO4 | MnSO4+Na2CO3→ AgNO3+KBr→ | ZnOH++OH‾=Zn(OH)2 Sn2+ + S2Ц= SnS | ||
| Cu(OH)2 Zn(NO3)2 | Al2(SO4)3+Na3PO4→ PbOHNO3+NaOH→ | 2Ag++CO32Ц=Ag2CO3 Mn2++2OH‾=Mn(OH)2 | ||
| Ba(OH)2 NH4H2PO4 | SnSO4+Na2S→ FeOHCl+NaOH→ | 2Sn2++2PO43Ц=Sn3(PO4)2 Cd2++2OH‾=Cd(OH)2 | ||
| H2S Ca(NO3)2 | Ni(NO3)2+Na2CO3→ BaBr2+CdSO4→ | 3Co2++2PO43Ц=Co3(PO4)2 Sn2++2OH‾=Sn(OH)2 | ||
| ZnOHNO3 Ca(OH)2 | Pb(NO3)2+Na3PO4→ NiCl2+NaOH→ | Ba2++SO32Ц=BaSO3 2Ag++S2Ц=Ag2S | ||
| Fe(OH)2NO3 Cr(NO3)3 | AgF+Na3PO4→ Al2(SO4)3+K2S→ | 2Fe3++3S2Ц=Fe2S3 Ni2++CO32Ц=NiCO3 | ||
| ZnOHCl Cu(NO3)2 | BaCl2+Al2(SO4)3→ PbS+HCl→ | Ag++Br‾=AgBr Al3++PO43Ц=AlPO4 | ||
| Fe(OH)2 Al2(SO4)3 | CuSO4+NaOH→ Ba(NO3)2+Na3PO4→ | Pb2++S2Ц=PbS Mn2++CO32‾=MnCO3 | ||
| Al(OH)2NO3 NaHS | AgNO3+K2S→ KHSO3+KOH→ | 3Ni2++2PO43Ц=Ni3(PO4)2 Zn2++S2Ц=ZnS | ||
| NH4HCO3 NiSO4 | AlCl3+K3PO4→ ZnOHNO3+NaOH→ | Ag++J ‾=AgJ Fe2++CO32‾=FeCO3 | ||
| H3BO3 Pb(NO3)2 | BaCl2+Na2CO3→ NiSO4+K2S→ | Al3++3OH‾=Al(OH)3 3Cd2++2PO43Ц=Cd3(PO4)2 | ||
онтрольное задание є 6
“ема: Ђ√идролиз солейї
«апишите уравнени€ гидролиза солей в молекул€рной и ионной формах, укажите рЌ раствора.
| є ва- рианта. | ‘ормулы солей | єва- рианта | ‘ормулы солей |
| Cu(NO3)2, K2SO3 | Na3PO4, Pb(NO3)2 | ||
| CH3COOK, ZnCl2 | Fe2(SO4)3, HCOOK | ||
| AlBr3, K2SiO3 | (CH3COO)2Ba, MnBr2 | ||
| K3PO4, Cr2(SO4)3 | Cd(NO3)2, HCOONH4 | ||
| FeJ2, HCOONa | SrS, CoBr2 | ||
| Ca(NO2)2, MnSO4 | (NH4)2SO4, K2CO3 | ||
| NiBr2, NH4F | (CH3COO)2Sr, SnJ2 | ||
| KClO, CdCl2 | Al(NO3)3, NH4ClO | ||
| Co(NO3)2, CH3COONH4 | (CH3COO)2Pb, FeCl3 | ||
| Na2SO3, NH4J | SnSO4, NaClO | ||
| AgNO3, BaS | (CH3COO)2Mn, NH4NO3 | ||
| (NH4)2S, SnCl2 | CoCl2, Na2SiO3 | ||
| CuSO4, BeS | CaS, CrBr3 | ||
| KNO2, ZnBr2 | Al2(SO4)3, NH4NO2 | ||
| AlCl3, K2S | (CH3COO)2Cu, NH4Cl |
онтрольное задание є 7
“ема: ЂOкислительно-востановительные реакцииї
”равн€ть электронно-ионным методом, указать окислитель и восстановитель:
| є вар | ”равнени€ реакций |
| CrCl3+Br2+KOH→K2CrO4+KBr+KCl+H2O KJ+KJO3+H2SO4→J2+K2SO4+H2O | |
| MnO2+KBr+H2SO4→MnSO4+Br2+K2SO4+H2O NaNO3+Cu+H2SO4→CuSO4+NO+Na2SO4+H2O | |
| FeSO4+HJO3+H2SO4→Fe2(SO4)3+J2+H2O Cr2O3+KNO3+KOH→K2CrO4+KNO2+H2O | |
| KMnO4+CO+H2SO4→MnSO4+CO2+K2SO4+H2O Mg+HNO3→Mg(NO3)2+N2+H2O | |
| K2Cr2O7+SO2+H2SO4→Cr2(SO4)3+K2SO4+H2O PbS+HNO3→S+Pb(NO3)2+NO+H2O | |
| Fe2O3+KNO3+KOH→K2FeO4+KNO2+H2O K2MnO4+Cl2→KMnO4+KCl | |
| K2Cr2O7+K2S+H2SO4→Cr2(SO4)3+S+K2SO4+H2O Mg+H2SO4→MgSO4+S+H2O | |
| KNO3+KJ+H2SO4→NO+J2+K2SO4+H2O K2Cr2O7+K2SO3+H2SO4→Cr2(SO4)3+K2SO4+H2O | |
| NaBrO3+NaBr+H2SO4→Br2+Na2SO4+H2O CuJ2+KMnO4+H2SO4→J2+MnSO4+CuSO4+K2SO4+H2O | |
| J2+Cl2+H2O→HJO3+HCl Zn+KJO3+H2SO4→ZnSO4+J2+K2SO4+H2O | |
| K2Cr2O7+KJ+H2SO4→Cr2(SO4)3+J2+K2SO4+H2O H2S+Cl2+H2O→H2SO4+HCl | |
| NaBrO3+F2+NaOH→NaBrO4+NaF+H2O KMnO4+FeSO4+H2SO4→MnSO4+Fe2(SO4)3+K2SO4+H2O | |
| K2CrO4+HCl→CrCl3+Cl2+KCl+H2O HClO4+SO2+H2O→HCl+H2SO4 | |
| KMnO4+K2S+H2O→MnO2+S+KOH Zn+HNO3→Zn(NO3)2+NH4NO3+H2O | |
| FeSO4+HNO3+H2SO4→Fe2(SO4)3+NO+H2O K2Cr2 O7+Al+H2SO4→Cr2(SO4)3+Al2(SO4)3+K2SO4+H2O |
| KMnO4+H2S+H2SO4→MnSO4+S+K2SO4+H2O K2Cr2O7+SnCl2+H2SO4→CrCl3+Sn(SO4)2+K2SO4+H2O | ||||
| MnO2+K2O+H2SO4→MnSO4+O2+K2SO4+H2O KMnO4+K2S+H2SO4→MnSO4+S+K2SO4+H2O | ||||
| KMnO4+HCl→MnCl2+Cl2+KCl+H2O Zn+H2SO4→ZnSO4+H2S+H2O | ||||
| K2Cr2O7+H2S+H2SO4→Cr2(SO4)3+S+K2SO4+H2O K2Se+NaNO3→K2SeO4+NaNO2 | ||||
| KMnO4+HNO2→Mn(NO3)2+KNO2+KNO3+H2O Cr2(SO4)3+Cl2+KOH→K2CrO4+KCl+K2SO4+H2O | ||||
| As2O3+HOCl+H2O→H3AsO4+HCl K2Cr2O7+NaNO2+H2SO4→Cr2(SO4)3+NaNO3+K2SO4+H2O | ||||
| K2MnO4+KJ+H2SO4→MnSO4+J2+K2SO4+H2O Mg+H2SO4→MgSO4+S+H2O | ||||
| K2CrO4+HCl→CrCl3+Cl2+KCl+H2O KMnO4+KNO2+H2O→KNO3+MnO2+KOH | ||||
| Na2CrO4+NaJ+H2SO4→Cr2(SO4)3+J2+Na2SO4+H2O Cu+HNO3→Cu(NO3)2+NO+H2O | ||||
| Cr2O3+KClO3+KOH→K2CrO4+KCl+H2O As2O3+J2+H2O→As2O5+HJ | ||||
| KMnO4+FeCO3+H2SO4→MnSO4+Fe2(SO4)3+CO2+K2SO4+H2O KJ+NaOCl+H2SO4→J2+NaCl+K2SO4+H2O | ||||
| KMnO4+SO2+KOH→K2MnO4+K2SO4+H2O K2Cr2O7+HCl→Cl2+CrCl3+KCl+H2O | ||||
| H2SO3+HClO3→H2SO4+HCl NaCrO2+Br2+NaOH→Na2CrO4+NaBr+H2O | ||||
| MnSO4+PbO2+HNO3→HMnO4+PbSO4+Pb(NO3)2+H2O S+HNO3→H2SO4+NO | ||||
| Zn+KNO2+KOH+H2O→K2[Zn(OH)4]+NH3 Co+HNO3→Co(NO3)2+N2+H2O | ||||
онтрольное задание є 8
“ема Ђ√альванический элементї
–ассмотреть работу гальванического элемента по плану:
1. ќпределите потенциалы электродов гальванического элемента.
2. ”становите анод и катод. «апишите процессы, протекающие на аноде и катоде.
3. —делайте условную графическую запись гальванического элемента, укажите в ней зар€ды электродов, направление движени€ электронов и ионов.
4. ќпределите Ёƒ— гальванического элемента.
| є варианта | —хема гальванического элемента | онцентраци€ электролита |
| Cu/CuCl2||CdCl2/Cd | CCu2+=0.1 M, CCd2+=0,002 M | |
| Ag/AgNO3||Zn(NO3)2/Zn | CAg+=0,2M, CZn2+=0,001M | |
| Pb/Pb(NO3)2||Mg(NO3)2/Mg | CPb2+=0,1 M, CMg2+=10-4 M | |
| Al/Al2(SO4)3||SnSO4.Sn | CAl3+0,01 M, CSn2+=0,5 M | |
| Fe/FeCl2||CoCl2/Co | CFe2+=0,2 M, CCo2+=0,001 M | |
| Ni/NiSO4||CuSO4/Cu | CNi2+=0,0001M, CCu2+=0,5M | |
| Ag/AgNO3||Cd(NO3)2/Cd | CAg+=0,1 M, CCd2+=0,002M | |
| Sn/Sn(NO3)2||Zn(NO3)2/Zn | CSn2+=0,0005 M, CZn2+=0,1 M | |
| Pb/Pb(NO3)2||Fe(NO3)2/Fe | CPb2+=0,005 M, CFe2+=0,2 M | |
| Cu/CuSO4||CoSO4/Co | CCu2+=2 M, CCo2+=10-3 M | |
| Ag/AgNO3||Ni(NO3)2/Ni | CAg+=0,001 M, CNi2+=0,5 M | |
| Sn/SnCl2||CoCl2/Co | CSn2+=0,1 M, CCo2+=0,005 M | |
| Pb/Pb(NO3)2||Cd(NO3)2/Cd | CPb2+=0,01 M, CCd2+=0,5 M | |
| Al/Al2(SO4)3||H2SO4/H2(Pt) | CAl3+=0,2 M, pH=2 | |
| Cu/CuCl2||MgCl2/Mg | CCu2+=0,001 M, CMg2+=2 M | |
| Zn/ZnCl2||AuCl3/Au | CZn2+=0,5 M, CAu3+=0,0001 M | |
| Ag/AgNO3||Fe(NO3)2/Fe | CAg+=0,0001 M, CFe2+=2 M | |
| Pb/Pb(NO3)2||Ni(NO3)2/Ni | CPb2+=0,1 M, CNi2+=0,001 M | |
| Sn/SnSO4||MgSO4/Mg | CSn2+=0,5 M, CMg2+=0,01 M | |
| Cu/CuCl2||ZnCl2/Zn | CCu2+=2 M, CZn2+=10-4 M | |
| Ag/AgNO3||Co(NO3)2/Co | CAg+=0,5 M, CCo2+=0,001 M | |
| Al/Al2(SO4)3||Au2(SO4)3/Au | CAl3+=10-4 M, CAu3+=2 M | |
| Pt/PtCl2||HCl/H2(Pt) | CPt2+=0,5 M, pH=1,5 M | |
| Sn/SnCl2||Pb(NO3)2/Pb | CSn2+=10-5 M, CPb2+=0,5 M | |
| Co/CoSO4||ZnSO4/Zn | CCo2+=0,2 M, CZn2+=0,001 M | |
| Ag/AgNO3||HNO3/H2(Pt) | CAg+=2 M, pH=1 | |
| (Pt)H2/H2SO4||H2SO4/H2(Pt) | pH=2,5, pH=3,5 | |
| Ag/AgNO3||Mg(NO3)2/Mg | CAg+=0,1 M, CMg2+=0,5 M | |
| Zn/ZnSO4||H2SO4/H2(Pt) | CZn2+=0,2 M, pH=3 | |
| Sn/SnCl2||FeCl2/Fe | CSn2+=0,01 M, CFe2+=0,05 M |
онтрольное задание є 9
“ема: ЂЁлектролизї
–ассмотреть электролиз водного раствора соли по плану:
1. «апишите все возможные процессы на аноде, установите потенциалы процессов.
2. —равните потенциалы анодных процессов и определите, какой из них протекает в первую очередь?
3. ¬ы€сните, мен€етс€ ли среда около анода, если да, то как и почему?
4. «апишите все возможные процессы на катоде, установите потенциалы процессов.
5. —равните потенциалы катодных процессов и определите, какой из них протекает в первую очередь.
6. ”становите, мен€етс€ ли среда около катода, если да, то как и почему?
7. «апишите итоговую схему процесса электролиза.
| є варианта | —остав и концентраци€ электролита | рЌ электролита и материал электродов |
| 0,1 M раствор Zn(NO3)2 | pH=4, катод Ц Zn, анод Ц — | |
| 0,5 M раствор MgBr2 | pH=6,5, электроды Ц Pt | |
| 0,1 M раствор NiSO4 | pH=5, электроды Ц Ni | |
| 0,1 M раствор FeJ2 | pH = 4,5, катод Ц Fe, анод Ц Pt | |
| 2 M раствор KNO2 | pH = 8, электроды Ц Pt | |
| 1 M раствор K2SO4 | pH = 7, катод Ц Fe, анод Ц Cu | |
| 0,01 M раствор Au(NO3)3 | pH = 6, катод Ц Au, анод Ц Pt | |
| 2 M раствор K4[Fe(CN)6] | pH = 7, электроды Ц Pt | |
| 0,5 M раствор CoCl2 | pH = 6,5, катод Ц Fe, анод Ц C | |
| 0,1 M раствор CuSO4 | pH = 5, катод Ц Al, анод Ц —u | |
| 0,01 M раствор FeF3 | pH = 6, электроды Ц C | |
| 2 M раствор Cr(NO3)3 | pH = 5, катод Ц Ni, анод Ц Cr | |
| 0,5 M раствор K2SO4 | pH = 6,5, катод Ц Fe, анод Ц Sn | |
| 1 M раствор AgNO3 | pH = 7, катод Ц Cu, анод Ц Ag | |
| 0,001 M раствор HCl | pH = 3, катод Ц Sn, анод Ц Cu | |
| 0,01 M раствор MnCl2 | pH = 6, катод Ц Mn, анод Ц Pt | |
| 0,2 раствор SnCl2 | pH = 5, катод Ц Fe, анод Ц Sn | |
| 0,001 M раствор ZnCl2 | pH = 6,5, катод Ц C, анод Ц Zn | |
| 0,0 M раствор MgCl2 | pH = 7, катод Ц Mg, анод Ц Pt | |
| 0,01 M раствор K3PO4 | pH = 10, электроды Ц C | |
| 0,2 M раствор ZnSO4 | pH = 5, электроды Ц Zn | |
| 0,01 M раствор KBr | pH = 8, катод Ц C, анод Ц Ni |
«адачи дл€ вариантов
3. —колько кулонов электричества необходимо пропустить через раствор Bi(NO3)3, чтобы на катоде выделилось 0,2 г висмута? «апишите процессы на катоде, аноде и суммарный процесс электролиза.
6. ѕри прохождении тока силой 1,5 ј в течении 30 минут через раствор соли трехвалентного металла на катоде выделилось 1,07 г металла. акой это металл?
9. „ерез раствор CuSO4 при рЌ=6 было пропущено 9650 кулонов электричества, при этом на катоде выделилось 2,86 г меди. ќпределите выход по току. «апишите суммарный процесс электролиза.
12. „ему равна толщина сло€ цинкового покрыти€, полученного в течении 1,5 часов при плотности тока 0,75 ј/дм2 и выходе по току 90% (плотность цинка составл€ет 7*103 г/дм3)?
15. ќпределите, сколько граммов гидроксида натри€ образовалось у катода при электролизе раствора хлорида натри€, если на катоде выделилось 2,8 л водорода, измеренного при нормальных услови€х? «апишите суммарный процесс электролиза.
18. –ассмотрите схему электрохимической очистки серебра, имеющего примеси золота, меди, никел€. уда помещают стержни с загр€зненным серебром? Ќа каком электроде получают чистое серебро? „то выступает электролитом? «апишите процессы на катоде и на аноде.
21. ѕри электролизе раствора нитрата никел€ на аноде выделилось 1120 мл кислорода, измеренного при нормальных услови€х. —колько граммов никел€ выделилось на катоде, если выход по току равен 70%? «апишите суммарный процесс электролиза.
24. „ерез раствор хлорида олова SnCl2 пропущено 10 ј×час электричества, при этом на катоде выделилось 18 граммов олова. Ќапишите уравнени€ реакций, протекающих на электродах. –ассчитайте выход олова по току.
онтрольное задание є 10
“ема: Ђ оррози€ металловї
–ассмотреть возможность коррозии сплава в заданной среде при доступе воздуха по плану:
1. ќпределить анод и катод в паре.
2. «аписать процессы протекающие на анодных и катодных участках.
3. –ассчитать потенциалы катодных процессов.
4. ќпределить возможность коррозии.
| є вар. | —плав | рЌ | є вар. | —плав | рЌ |
| Fe-Ni | 10 | Cu-Al | 10 | ||
| Cd-Sn | 7 | Fe-Ni | 5 | ||
| —o-Cu | 5 | Pb-Sn | 7 | ||
| Fe-Pb | 10 | Ag-Au | 10 | ||
| Cd-Ni | 7 | Fe-Mn | 5 | ||
| Cu-Pb | 5 | Al-Mg | 7 | ||
| Fe-Co | 10 | Cu-Ag | 10 | ||
| Co-Ni | 7 | Sn-Pb | 5 | ||
| Mg-Fe | 5 | Zn-Cd | 7 | ||
| Zn-Pb | 10 | Ag-Ni | 10 | ||
| Au-Ni | 7 | Fe-Bi | 12 | ||
| Mg-Ni | 5 | Bi-Sn | 4 | ||
| Ni-Sn | 10 | Bi-Pb | 2 | ||
| Co-Pb | 7 | Cd-Fe | 11 | ||
| Cd-Ag | 5 | Al-Mg | 9 |
онтрольное задание є 11
“ема: Ђ—войства металловї
Ќаписать реферат по следующему плану:
1. Ёлектронна€ конфигураци€ атома. ¬озможные степени окислени€.
2. Ќахождение в природе и получение в свободном виде.
3. ‘изические и химические свойства.
4. —войства соединений.
5. —плавы. ѕрименение металла и его соединений.
| є вар. | ћеталл | є вар. | ћеталл | є вар. | ћеталл |
| Ѕериллий | ќлово |
|
|
|
|
|
|
ƒата добавлени€: 2016-11-24; ћы поможем в написании ваших работ!; просмотров: 1293 | Ќарушение авторских прав
Ћучшие изречени€:
√ен: 0.16 с.






