При действии H2S+ H2O или Na2S2O3 на соли висмута при нагревании выпадает черно-бурый сульфид висмута:
2Bi3+ + 3H2S = Bi2S3 ↓+ 6H+,
2Bi3+ + 3S2O32- + 3H2O = Bi2S3 ↓+ 6H+ + 3SO42-
Выполнение реакции. К раствору Bi(NO3)3 добавить несколько капель 2н H2SO4 и несколько кристалликов Na2S2O3. Подогреть, наблюдать выпадение сульфида висмута. Провести опыт с сероводородной водой.
ХАРАКТЕРНЫЕ РЕАКЦИИ КАТИОНА Sb3+
Сурьма образует 2 ряда соединений, в которых проявляет степени окисления +3 и +5. Растворы солей сурьмы бесцветны.
Гидролиз.
При действии воды на соли сурьмы они подвергаются гидролизу по уравнению:
Sb3+ + H2O + Cl¯ = SbOCl↓ + 2H+
Нагревание способствует образованию осадка, добавление винной кислоты или сегнетовой соли – препятствует, в отличие от соединений висмута.
Выполнение реакции. В пробирку к нескольким каплям хлорида сурьмы (III) добавить 2-3 кратный избыток воды. Наблюдать выпадение осадка хлористого стибила. Сравнить степень гидролиза солей сурьмы и висмута. Указать пути усиления и уменьшения степени гидролиза. Проверить действие растворов HCl и винной кислоты на осадок. Сделать вывод о возможности разделения катионов сурьмы и висмута с помощью гидролиза.
- Реакция с сероводородной водой.
При пропускании H2S или добавлении H2S+ H2O в солянокислый раствор Sb(III), Sb(V) выпадает оранжевый осадок сульфида:
2Sb3++ 3 H2S= Sb2S3 ↓+ 6H+
2Sb5+ + 5 H2S= Sb2S5 ↓+ 10 H+
Осадки растворяются в избытке Na2S или NaOH при нагревании.
Выполнение реакции. В пробирку с 5-7 каплями SbCl3 добавить равный объём насыщенного раствора H2S. Описать наблюдаемые явления.
- Реакция с тиосульфатом натрия.
Na2S2O3 даёт с катионом Sb3+ при нагревании красный осадок сероокиси сурьмы Sb2OS2 (сурьмяная киноварь):
2Sb3++2 S 2O32- + 3 H2O= Sb2OS2 ↓+ 2 SO42- +6H+
При наличии Bi3+ выпадает черный осадок, который будет маскировать окраску Sb2OS2. Мешают проведению реакции Cu2+, Hg2+ и другие ионы, образующие малорастворимые сульфиды, а так же большой избыток кислоты, растворяющий реагент с выделением SO2 и S, мешающих проведению реакции.
Выполнение реакции. В пробирку с 3-4 каплями SbCl3 добавить каплю раствора H2SO4 и 1-2 кристаллика Na2S2O3. Нагреть. Что происходит?
- Реакция с Zn, Al, Fe.
Активные металлы восстанавливают Sb3+ до металлической сурьмы в кислой среде:
3Zn + 2[SbCl6]3- = 2Sb + 3Zn2+ + 12Cl-
Выполнение реакции. На хорошо очищенную наждачной бумагой цинковую, алюминиевую или железную пластинку нанести каплю подкисленной анализируемой соли. Через 2-3 минуты поверхность пластинки должна под каплей почернеть.
- Реакция с метиловым фиолетовым.
При добавлении к солянокислому раствору хлорида сурьмы растворов KNO2 или NaNO2 происходит окисление [SbCl6]3- в [SbCl6]¯. С метиловым фиолетовым, находящимся в кислом растворе в виде катиона, образуется ионная пара [B]+ [SbCl6]-, хорошо экстрагируемая бензолом, при этом органический слой окрашивается в синий цвет. В отсутствии Sb(V) раствор имеет желто- зелёную окраску.
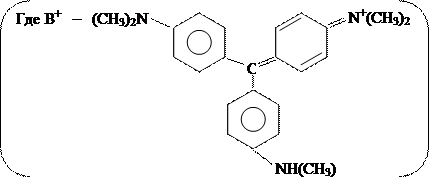 |
Открытие сурьмы этой реакцией возможно в присутствии Sn2+, Fe3+, Zn2+, Cu2+ в количествах, превышающих содержание Sb>1000 раз.
Выполнение реакции. К капле исследуемого раствора добавить 1-2 капли концентрированной соляной кислоты, кристаллик KNO2, 1-2 капли 0,2% реагента и 0,5 мл бензола. Встряхнуть, что наблюдается?






