711. √альванические элементы Ц устройства, преобразующие химическую энергию в электрическую. ѕростейшим гальваническим элементом €вл€етс€ элемент ƒаниэл€-якоби, разработанного в 1836 году. Ёлемент ƒаниэл€-якоби представл€ет собой медно-цинковый элемент. ћедный и цинковый электроды погружены в растворы своих солей и образуют полуэлементы, их разграничивает пориста€ перегородка, преп€тствующа€ смешению растворов, но пропускающа€ сульфат-анионы.
анод (-) Zn|ZnSO4, H2O||CuSO4 , H2O |Cu(+) катод
(-) Zn Ц 2e- = Zn2+
(+) Cu2+ + 2e- = Cu
Zn + Cu2+ = Zn2+ + Cu
»сточником электрического тока при работе этого элемента €вл€етс€ пространственно разделенна€ окислительно-восстановительна€ реакци€ вытеснени€ более активного металла менее активным из его соли.
712. ѕри работе гальванических элементов стандартными называют услови€ “ = 298 , – = 1.013*105 ѕа, концентраци€ катионов соли в растворе Ц 1 г-ион/л. Ёлемент ƒаниэл€-якоби представл€ет собой медно-цинковый элемент. ћедный и цинковый электроды погружены в растворы своих солей и образуют полуэлементы, их разграничивает пориста€ перегородка, преп€тствующа€ смешению растворов, но пропускающа€ сульфат-анионы.
анод (-) Zn|ZnSO4, H2O||CuSO4 , H2O |Cu(+) катод
(-) Zn Ц 2e- = Zn2+
(+) Cu2+ + 2e- = Cu
Zn + Cu2+ = Zn2+ + Cu
Ёƒ— = E0(Cu2+/Cu) - E0(Zn2+/Zn) = 0.34 Ц (-0.76) = 1.10 ¬.
713. Ёлектрод Ц металлическа€ пластина,контактирующа€ с электролитом, €вл€юща€с€ передатчиком электронов от восстановител€ к внешнему проводнику и от внешнего проводника к окислителю. ѕоложительно зар€жен катод, на котором протекает реакци€ восстановлени€ (Cu2+ + 2e- = Cu). ќтрицательно зар€жен анод, на котором протекает реакци€ окислени€ (Zn - 2e- = Zn2+).
714. ¬ основу работы гальванического элемента можно положить реакции: Zn + Cu2+ = Zn2+ + Cu, Fe + Pb2+ = Fe2+ + Pb, KMnO4 + FeSO4 + H2SO≠4 = MnSO≠4 + Fe2(SO4)3 + K2SO4 + H2O, K2Cr2O7 + K2SO3 + H2SO≠4 = Cr2(SO≠4)3 + K2SO4 + H2O.
анод (-) Zn|ZnSO4, H2O||CuSO4 , H2O |Cu(+) катод
(-) Zn Ц 2e- = Zn2+
(+) Cu2+ + 2e- = Cu
Zn + Cu2+ = Zn2+ + Cu
715. ¬ основе расчета Ёƒ— лежит термодинамический принцип.
анод (-) Zn|ZnSO4, H2O||CuSO4 , H2O |Cu(+) катод
(-) Zn Ц 2e- = Zn2+
(+) Cu2+ + 2e- = Cu
Zn + Cu2+ = Zn2+ + Cu
Ёƒ— = E0(Cu2+/Cu) - E0(Zn2+/Zn) = 0.34 Ц (-0.76) = 1.10 ¬.
(-)Zn|ZnSO4||AgNO3|Ag(+)
Cн(ZnSO4)=0.01н., Cн(AgNO3)=0.1н.,T = 27oC =300 K
(-) Zn Ц 2e- = Zn2+
(+) Ag1+ + 1e- = Ag Zn +2Ag1+ = Zn2+ + Ag
[Zn2+] = Cм(ZnSO4) = —н/2 = 0.01/2 = 0.005 моль/л
[Ag+] = Cм(AgNO3) = —н = 0.1 моль/л
Ёƒ— = E(Ag+/Ag) - E(Zn2+/Zn) = E0(Ag+/Ag) - E0(Zn2+/Zn) + 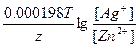 = -0.8 Ц (-0.76) +
= -0.8 Ц (-0.76) + 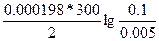 = 1.58B
= 1.58B
716.
| ƒано: Cн(ZnSO4)=0.01н. Cн(AgNO3)=0.1н. T = 27oC =300 K Ёƒ— =?. | (-)Zn|ZnSO4||AgNO3|Ag(+)
(-) Zn Ц 2e- = Zn2+
(+) Ag1+ + 1e- = Ag Zn +2Ag1+ = Zn2+ + Ag
[Zn2+] = Cм(ZnSO4) = —н/2 = 0.01/2 = 0.005 моль/л
[Ag+] = Cм(AgNO3) = —н = 0.1 моль/л
Ёƒ— = E(Ag+/Ag) - E(Zn2+/Zn) = E0(Ag+/Ag) - E0(Zn2+/Zn) + 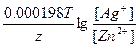 = -0.8 Ц (-0.76) + = -0.8 Ц (-0.76) + 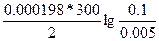 = 1.58B = 1.58B
|
|
|
|
717.
| ƒано: Cн(Mg(NO3)2)=0.2н. Cн(SnSO4) = 0.03н. T = 17oC = 290 K Ёƒ— =?. | (-)Mg|Mg(NO3)2||SnSO4|Sn(+)
(-) Mg Ц 2e- = Mg2+
(+) Sn2+ + 2e- = Sn Mg + Sn2+ = Mg2+ + Sn
[Mg2+] = Cм(Mg(NO3)2) = —н/2 = 0.2/2 = 0.1 моль/л
[Sn2+] = Cм(SnSO4) = —н/2 = 0.03/2 = 0.015 моль/л
Ёƒ— = E(Sn2+/Sn) - E(Mg2+/Mg) = E0(Sn2+/Sn) - E0(Mg2+/Mg) + 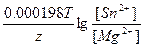 = -0.14 + 2.37 + = -0.14 + 2.37 + 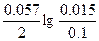 = 2.21 ¬. = 2.21 ¬.
|
718.
| ƒано: Cн(Fe(NO3)2)=0.03н. Cн(Pb(NO3)2)=0.1н. T = 37oC = 310 K Ёƒ— =?. | (-)Fe|Fe(NO3)2|| Pb(NO3)2|Pb(+)
(-) Fe Ц 2e- = Fe2+
(+) Pb2+ + 2e- = Pb Fe + Pb2+ = Fe2+ + Pb
[Fe2+] = Cм(Fe(NO3)2) = —н/2 = 0.03/2 = 0.015 моль/л
[Pb2+] = Cм(Pb(NO3)2) = —н/2 = 0.1/2 = 0.05 моль/л
Ёƒ— = E(Pb2+/Pb) - E(Fe2+/Fe) = E0(Pb2+/Pb) - E0(Fe2+/Fe) + 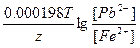 = -0.13 + 0.45 + = -0.13 + 0.45 + 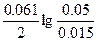 = 0.34 ¬. = 0.34 ¬.
|
719.
| ƒано: Cн(MnCl2)=0.01н. Cн(CdCl2)= 0.5н. T = 25oC = 298 K Ёƒ— =?. | (-)Mn|MnCl2||CdCl2|Cd(+)
(-) Mn Ц 2e- = Mn2+
(+) Cd2+ + 2e- = Cd Mn+ Cd2+=Mn2+ +Sn
[Mn+] = Cм(MnCl2) = Cн/2 = 0.01/2 = 0.005 моль/л
[Cd2+] = Cм(Cd—l2) = —н/2 = 0.05/2 = 0.025 моль/л
Ёƒ— = E(Cd2+/Cd) - E(Mn2+/Mn) = E0(Cd2+/Cd) - E0(Mn2+/Mn) + 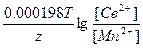 = -0.40 + 1.17 + = -0.40 + 1.17 + 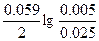 = 0.749 ¬. = 0.749 ¬.
|
720.
| ƒано: Cн(NiCl2) = 0.04н. Cн(CuCl2)= 0.08н. T = 65oC = 338 K Ёƒ— =?. | (-)Ni|NiCl2||CuCl2|Cu(+)
(-) Ni Ц 2e- = Ni2+
(+) Cu2+ + 2e- = Cu Ni + Cu2+ = Ni2+ + Cu
[Ni2+] = Cм(NiCl2) = —н/2 = 0.04/2 = 0.02 моль/л
[—u2+] = Cм(CuCl2) = —н /2= 0.08/2 = 0.04 моль/л
Ёƒ— = E(Cu2+/Cu) - E(Ni2+/Ni) = E0(Cu2+/Cu) - E0(Ni2+/Ni) + 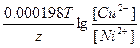 = 0.34 + 0.25 + = 0.34 + 0.25 + 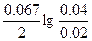 = 0.6 ¬. = 0.6 ¬.
|
721.
| ƒано: Cн(Cd(NO3)2)=0.06н. Cн(AgNO3) = 0.05н. T = 57oC = 330 K Ёƒ— =?. | (-)Cd|Cd(NO3)2||AgNO3|Ag(+)
(-) Cd Ц 2e- = Cd2+
(+) Ag+ + e- = Ag
Cd + 2Ag+ = Cd2+ + 2Ag Cd + 2AgNO3 = Cd(NO3)2 + 2Ag
[Cd2+] = Cм(Cd(NO3)2) = —н/2 = 0.06/2 = 0.03 моль/л
[Ag+] = Cм(AgNO3) = —н = 0.05 моль/л
Ёƒ— = E(Ag+/Ag) - E(Cd2+/Cd) = E0(Ag+/Ag) - E0(Cd2+/Cd) + 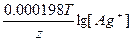 - - 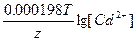 = 0.8 + 0.4 + = 0.8 + 0.4 + 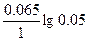 - - 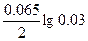 = 1.15 ¬ = 1.15 ¬
|
722.
| ƒано: Cн(Sn(NO3)2)=0.07н. Cн(Hg(NO3)2)=0.09н. T = 23oC = 290 K Ёƒ— =?. | (-)Sn|Sn(NO3)2||Hg(NO3)2|Hg(+)
(-) Sn Ц 2e- = Sn2+
(+) Hg2+ + 2e- = Hg
Sn + Hg2+ = Sn2+ + Hg Sn + 2Hg(NO3)2 = Sn(NO3)2 + Hg
[Sn2+]= Cм(Sn(NO3)2) = —н/2 = 0.07/2 = 0.035 моль/л
[Hg2+]= Cм(Hg(NO3)2) = —н/2 = 0.09/2 = 0.045 моль/л
Ёƒ— = E(Hg2+/Hg) - E(Sn2+/Sn) = E0(Hg2+/Hg) - E0(Sn2+/Sn) + 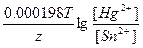 = 0.79 + 0.14 + = 0.79 + 0.14 + 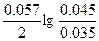 = 0.94 ¬ = 0.94 ¬
|
723.
| ƒано: Cн(Al2(SO4)3)=0.3н. Cн(Bi(NO3)3)=0.3н. T = 33oC = 310 K Ёƒ— =?. | (-)Al|Al2(SO4)3||Bi(NO3)3|Bi(+)
(-) Al Ц 3e- = Al3+
(+) Bi3+ + 3e- = Bi
Al + Bi3+ = Al3+ + Bi
[Al3+]= Cм(Al2(SO4)3) = —н/3 = 0.3/3 = 0.1 моль/л
[Bi3+]= Cм(Bi(NO3)3) = —н/3 = 0.3/3 = 0.1 моль/л
Ёƒ— = E(Bi3+/Bi) - E(Al3+/Al) = E0(Bi3+/Bi) - E0(Al3+/Al) + 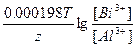 = 0.2 + 1.62 + = 0.2 + 1.62 + 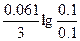 = 1.82 ¬ = 1.82 ¬
|
724.
| ƒано: Cн(CaCl2) = 0.5 н. Cн(H2SO4)=0.02н. T = 20oC = 293 K Ёƒ— =?. | (-)Ca|CaCl2||H2SO4|H2(Pt)(+)
(-) —a Ц 2e- = Ca2+
(+) 2H+ + 2e- = H2
Ca + 2H+ = Ca2+ + H2
[Ca2+] = Cн(CaCl2) = 0.5 н = 0.25 моль/л
[Ќ+] = 2Cн(H2SO4) = 0.04 н = 0.02 моль/л
Ёƒ— = E(H+/H2) - E(Ca2+/Ca) = E0(H+/H2) - E0(Ca2+/Ca) + 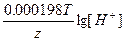 - - 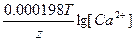 = 0 + 2.87 + = 0 + 2.87 + 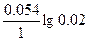 - - 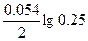 = 2.79 ¬. = 2.79 ¬.
|
725.
|
|
|
| ƒано: Cн(Li2SO4) = 0.1н Cн(AgNO3) = 0.1н T = 0oC = 273 K Ёƒ— =?. | (-)Li|Li2SO4||AgNO3|Ag(+)
(-) Li Ц 1e- = Li+
(+) Ag+ + 1e- = Ag Li + Ag+ = Li+ + Ag
[Li+] = 2Cм(Li2SO4) = —н = 0.1 моль/л
[Ag+] = Cм(AgNO3) = —н = 0.1 моль/л
Ёƒ— = E(Ag+/Ag) - E(Li+/Li) = E0(Ag+/Ag) - E0(Li+/Li) + 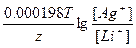 = 0.8 + 3.02 + = 0.8 + 3.02 + 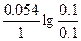 = 3.82 ¬. = 3.82 ¬.
|
726. √альванический элемент с максимальным значением Ёƒ—: (-)Li|Li2SO4||AuNO3|Au(+) Ёƒ— = Eo(Au+/Au) - Eo(Li+/Li) = 1.68+3.02 = 4.7 ¬
727.
| ј) ћедь Ц анод. (-)Cu|CuSO4||Ag2SO4|Ag(+) E = E0(Ag+/Ag) - E0(Cu2+/Cu) = 0.8 Ц 0.34 = 0.46 ¬. |
| Ѕ) ћедь Ц катод. (-)Fe|FeSO4||—uSO4|Cu(+) E = E0(Cu2+/Cu) - E0(Fe2+/Fe) - = 0.34 + 0.45 = 0.79 ¬. |
728. KMnO4 + FeSO4 + H2SO≠4 = MnSO≠4 + Fe2(SO4)3 + K2SO4 + H2O.
| (-)Fe|Fe2+,Fe3+||MnO4-, Mn2+|Fe(+) |
729. K2Cr2O7 + K2SO3 + H2SO≠4 = Cr2(SO≠4)3 + K2SO4 + H2O.
| Cr2O72-,Cr3+:φ0 = 1.33 B, SO32-, SO42-: φ0 = 0.17 B |
| (-)Pt| SO32-, SO42-, H+|| Cr2O72-,Cr3+, H+ |Pt(+) |
730.
| (-)Pt, H2| H+|| H+ | H2, Pt(+) |
| pH1 =4 pH2 = 2 |
pH = -lgC(H+), C1(H+) = 10-2 , C2(H+) = 10-4 . E0 (H2/H+) = 0 B.
E = 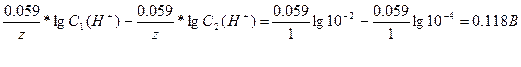
731. онцентрационные элементы Ц гальванические элементы, образованные одинаковыми электродами, опущенными в растворы своих солей разной концентрации.
| (-)Cu| CuSO4(C1 = 0.1%)|| CuSO4(C2 = 5.0%) | Cu (+) |
| Ёƒ— = (0.059/z)*lg(C2/C1) = (0.059/2)lg(5/0.1) = 0.05 ¬. |
732. “опливный элемент Ц это гальванический элемент, в котором в качестве активного материала отрицательного электрода используютс€ либо природное топливо (природный газ, содержащий углеводород), либо водород, окись углерода и др. восстановители. јктивным материалом положительного материала служит кислород. “окообразующа€ реакци€ сводитс€ к окислению топлива.
¬одород-кислородный “Ё разделен электродами на три плоскости Ц водородную, кислородную и промежуточную, заполненную электролитом. Ёлектроды изготовлены из пористого никел€, электролитом €вл€етс€ раствор ќЌ, водород и кислород подаютс€ под давлением 30-75 атм. Ёлемент работает при “=2000 —. —хема: (-)Ќ2| ќЌ, Ќ2ќ| O2(+)
јнод: 2Ќ2 Ц 4е- + 4ќЌ- = 4Ќ2ќ, катод: ќ2 + 4е- + 2Ќ2ќ = 4ќЌ-
ќбща€ реакци€: 2Ќ2 + ќ2 = 2Ќ2ќ.
733. јккумул€торы Ц химические источники тока, которые могут быть использованы многократно. »х работоспособность восстанавливаетс€ многократно, т.к. в основе их действи€ лежат химически обратимые процессы. —винцовый аккумул€тор состоит из чередующихс€ пластин, одна из которых представл€ет собой PbO2, а втора€ Ц металлический свинец в виде пористой губки. ѕластины погружены в 35-40% раствор серной кислоты. Ёлектрохимическа€ схема зар€женного аккумул€тора: (+)PbO2|H2SO4,H2O|Pb(-)
ѕри работе (разр€дке) протекают реакции: (+)Pb Ц2e - + SO42- = PbSO4 , (-)PbO2 +2e - + SO42- + 4H+ = PbSO4 + 2H2O.
Ёлектрохимическа€ схема разр€женного аккумул€тора: (-)PbSO4|H2SO4,H2O|PbSO4(+)
ѕротекают процессы: (-)PbSO4+2e - = Pb- + SO42, (+)PbSO4 -2e - + 2H2O.= PbO2+ + SO42- + 4H+
734.
| ƒано: E(Zn2+/Zn)=-0.81¬ C(ZnCl2) = 0.07 м. a(ZnCl2) =?. | E(Zn2+/Zn) = Eo(Zn2+/Zn) + 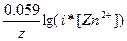 = -0.76 + (0.059/2) * lg(i*0.07) = -0.81, следовательно, i = 1.8. a = (i-1)(m-1) = (1.8-1)/(2-1) = 0.8 = 80%, где m Ц количество ионов, на которые диссоциирует электролит. = -0.76 + (0.059/2) * lg(i*0.07) = -0.81, следовательно, i = 1.8. a = (i-1)(m-1) = (1.8-1)/(2-1) = 0.8 = 80%, где m Ц количество ионов, на которые диссоциирует электролит.
|
735.
| ƒано: E(Ag+/Ag) = 0.59 ¬ S(Ag2CrO4) =?. | E(Ag+/Ag) = Eo(Ag+/Ag) + 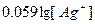 = 0.8 + 0.059*lg[Ag+] = 0.59, следовательно, [Ag+] = 2.8*10-4 м. S = = 0.8 + 0.059*lg[Ag+] = 0.59, следовательно, [Ag+] = 2.8*10-4 м. S = 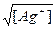 = 1.66*10-2 = 1.66*10-2
|
736.
| ƒано: E(Ag+/Ag)=0.51 ¬ ѕ–(Ag—l) =?. | E(Ag+/Ag) = Eo(Ag+/Ag) + 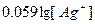 = 0.8 + 0.059*lg[Ag+] = 0.51, следовательно, [Ag+] = S = 1.2*10-5 м. ѕ– = S2 = 1.47*10-10 (S-растворимость) = 0.8 + 0.059*lg[Ag+] = 0.51, следовательно, [Ag+] = S = 1.2*10-5 м. ѕ– = S2 = 1.47*10-10 (S-растворимость)
|
737.
| (-)Mg|Mg2+||Ag+|Ag(+) E = E0(Ag+/Ag) - E0(Mg2+/Mg) = 0.8 + 2.37 = 3.17 B ΔG0 = -zFE = -2*96500*3.17 = -611.81 кƒж/моль ΔG0 = -RTlnKp = 8.31*298*lnKp = 611.81, следовательно, р = 1.28 |
738.
|
|
|
| (-)Fe|Fe2+||Cu2+|Cu(+) E = E0(Cu+2/Cu) - E0(Fe2+/Fe) = 0.34 - (-0.45) = 0.79 B ΔG0 = -zFE = -2*96500*0.79 = -152.5 кƒж/моль ΔG0 = -RTlnKp = 8.31*298*lnKp = 152.5, следовательно, р = 5.5*1026. |
739.
| ƒано: Cм(FeSO4)=1м. Ёƒ— = 0 ¬. Cм(CdSO4) =?. | (-)Fe|FeSO4||CdSO4|Cd(+)
Ёƒ— = E(Fe2+/Fe) - E(Cd2+/Cd) = E0(Fe2+/Fe) - E0(Cd2+/Cd) + 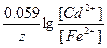 .
0= -0.40 + 0.45 + .
0= -0.40 + 0.45 + 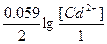 , следовательно, [Cd2+] = Cм(CdSO4) =0.02 м. , следовательно, [Cd2+] = Cм(CdSO4) =0.02 м.
|
Ёлектролиз
740. Ё ƒ— гальванических элементов вычисл€етс€ как разница между потенциалом катода и потенциалом анода, например, дл€ элемента (-)Fe|FeSO4||CdSO4|Cd(+) Ёƒ— = E0(Fe2+/Fe) - E0(Cd2+/Cd) + 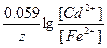 . ¬идно, что к увеличению Ёƒ— приведет увеличение концентрации катионов у катода и уменьшение концентрации катионов у анода, а к уменьшению ЁЋ— Ц наоборот.
. ¬идно, что к увеличению Ёƒ— приведет увеличение концентрации катионов у катода и уменьшение концентрации катионов у анода, а к уменьшению ЁЋ— Ц наоборот.
741. Ёлектролиз Ц окислительно-восстановительные процессы, протекающие в расплавах и растворах под действием электрического тока. ”стройство дл€ осуществлени€ электролиза называетс€ электролизером. Ёлектролизер представл€ет собой ванну, в которую погружены электроды: анод и катод.
    (-) атод эл. ток јнод (+) (-) атод эл. ток јнод (+)
электролит
|
ѕример: электролиз расплава хлорида натри€. атодный процесс Ц восстановление ионов натри€ до металлического натри€: Na+ + 1e = Na, анодный процесс Ц окисление ионов хлора до свободных атомов и рекомбинаци€ последних в молекулы: 2—l- -2e = Cl2.
742. ѕроцессы электролиза начинают протекать при достижении определенного внешнего напр€жени€, называемого напр€жением разложени€. Ќапр€жение разложени€ численно равно Ёƒ— гальванического элемента, образующегос€ при электролизе в результате выделени€ продуктов на электродах. ≈ще напр€жение разложени€ можно определить экспериментально. ѕример: электролиз расплава хлорида натри€. атод покрываетс€ слоем натри€, анод Ц слоем хлора, поэтому их можно рассматривать как натриевый и хлорный электроды, образующие гальваническую цепь. (-)Na|NaCl|Cl(+). —тандартный электродный потенциал натри€ равен Ц2.71 ¬, а хлора +1.36¬, поэтому Ёƒ— образованного ими элемента равна 4.07¬. —ледовательно, чтобы вызвать электролиз, следует приложить внешнее напр€жение не менее 4.07¬.
743. ѕроцессы электролиза начинают протекать при достижении определенного внешнего напр€жени€, называемого напр€жением разложени€ (перенапр€жени€). Ќапр€жение разложени€ численно равно Ёƒ— гальванического элемента, образующегос€ при электролизе в результате выделени€ продуктов на электродах. явление перенапр€жени€ нежелательно потому, что оно приводит к повышенному расходу электроэнергии. »ногда это €вление оказываетс€ полезным и позвол€ет провести такие процессы, которые без перенапр€жени€ не ведут к желаемому результату. Ќапример, гальваническое хромирование возможно потому, что нар€ду с вли€нием концентрации электролита вли€ние на процесс электролиза оказывает еще и перенапр€жение водорода.
744. ≈сли электродный потенциал металла гораздо меньше водородного потенциала, то на катоде выдел€етс€ только водород. ≈сли электродный потенциал металла гораздо больше водородного потенциала, то на катоде выдел€етс€ только металл. ≈сли электродный потенциал металла (Sn, Pb и др.) в растворе близок к водородному, то на катоде одновременно выдел€ютс€ металл и водород.
745. »з анионов в водных растворах электролитов при электролизе не разр€жаютс€ анионы кислородных кислот (SO42- , NO3- и др.), т.к. электроны в них св€заны прочнее и вместо них на аноде разр€жаютс€ ќЌ- или молекулы воды: 4ќЌ- + 4е - = 2Ќ2ќ + ќ2, 2Ќ2ќ Ц 4е - = 4Ќ+ + ќ2. ¬ обоих случа€х на аноде выдел€етс€ кислород. ќкисление самого анода происходит в том случае, когда анод содержит те же катионы, которые содержатс€ в растворе соли.
|
|
|
746. –азр€д катионов металлов на катоде при электролизе сопровождаетс€ присоединением электронов, следовательно, катионы металлов про€вл€ют в этом процессе окислительную способность. »сход€ из свойств р€да напр€жений, в первую очередь будут разр€жатьс€ самые неактивные металлы - сначала золото, затем платина и т.д. ≈сли электролиз ведетс€ в расплаве, то после меди разр€жаютс€ катионы свинца, затем олова, никел€ и т.д. до кали€. ќсобенностью электролиза растворов €вл€етс€ наличие в них, нар€ду с ионами электролита, ионов водорода за счет диссоциации воды. ¬ этом случае неактивные металлы (Cu-Au), сто€щие в р€ду напр€жений после водорода легко восстанавливаютс€ из растворов, а активные металлы (Na-Al) не восстанавливаютс€ совсем. ¬место них на катоде идет разр€дка ионов водорода (если среда кисла€) 2Ќ+ + е- = Ќ2 или молекул воды (нейтральна€ или щелочна€) 2Ќ2ќ + 2е- = 2ќЌ- + Ќ2. ћеталлы, расположенные между алюминием и водородом (Mn-Pb) за счет изменени€ потенциала водорода в воде, также восстанавливаютс€ из растворов. »з анионов в водных растворах в первую очередь разр€жаютс€ анионы бескислородных кислот Cl-, Br-, I-, S2- и другие, (кроме F-). ¬ анионах кислородных кислот электроны св€заны прочнее, поэтому вместо них на аноде разр€жаютс€ анионы ќЌ- в щелочной среде 4ќЌ- - 4е- = 2Ќ2ќ + ќ2, или молекулы воды в нейтральной и кислой средах 2Ќ2ќ Ц 4е- = 4Ќ+ + ќ2. јнионы, не разр€жающиес€ при электролизе растворов, разр€жаютс€ при электролизе расплавов. ѕри этом из расплавленных фторидов на аноде выдел€етс€ газообразный фтор, а при электролизе сульфатов, нитратов и т.п. различные продукты, состав которых зависит от условий проведени€ процесса.
747. оличественна€ характеристика электролиза описываетс€ формулами: 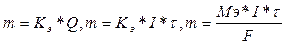 , где m - граммы,
, где m - граммы, 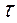 - секунды, F = 96500, I - амперы, ћэ - г/моль. Ёлектролиз CuSO4 + H2O = Cu + H2SO4 + 1/2O2
- секунды, F = 96500, I - амперы, ћэ - г/моль. Ёлектролиз CuSO4 + H2O = Cu + H2SO4 + 1/2O2
| атод: Cu2+ + 2e- = Cu јнод: H2O Ц 2e- = 2H+ + 1/2O2 | m(Cu) = 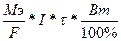 = (32*20*12*3600*90%)/(96500*100%) = 258.7 грамм = (32*20*12*3600*90%)/(96500*100%) = 258.7 грамм
|
748. ѕриведенные формулы идентичны, поскольку 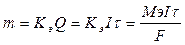 . ‘ормулы идентичны при размерност€х: m - граммы,
. ‘ормулы идентичны при размерност€х: m - граммы, 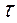 - секунды, F = 96500, I - амперы, ћэ - г/моль.
- секунды, F = 96500, I - амперы, ћэ - г/моль.
749. ѕосто€нна€ ‘араде€ F = 96487 л Ц количество электричества, выдел€ющее при электролизе один грамм-эквивалент любого вещества. Ёлектрохимический эквивалент э численно равен массе вещества, выделившейс€ при действии одного кулона электричества. э = Ё/F. ƒл€ меди э = 32/96487 = 3.32*10-4 г., дл€ серебра э = 108/96487 = 1.12*10-3 г., дл€ NaOH э = 40/96487 = 4.14*10-4 г.
750. «атраченное на электролиз электричество расходуетс€ не только на целевую реакцию, но и на побочные процессы Ц окисление примесей и др. ѕоэтому масса вещества оказываетс€ меньше теоретически рассчитанной. ќтношение масс называетс€ выходом по току.
751. Ёлектролиз CuSO4 + H2O = Cu + H2SO4 + 1/2O2
| атод: Cu2+ + 2e- = Cu јнод: H2O Ц 2e- = 2H+ + 1/2O2 | m(Cu) = 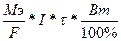 = (32*20*12*3600*90%)/(96500*100%) = 258.7 грамм = (32*20*12*3600*90%)/(96500*100%) = 258.7 грамм
|
752. Ёлектролиз ZnSO4
| атод: Zn2+ + 2е = Zn, Aнод: Ќ2ќ Ц 2е - = 2Ќ+ + 0.5ќ2. —уммарна€ реакци€: ZnSO4 + Ќ2ќ = Zn + Ќ2SO4 + 0.5ќ2 | |
| ƒано t = 10 часов = 36000 сек. ¬т = 50%, I = 50 ј. m(Zn) =? | m(Zn) = 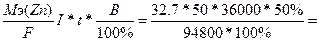 310 грамм 310 грамм
|
753. Ёлектролиз FeSO4
| атод: Fe2+ + 2е = Fe, Aнод: Ќ2ќ Ц 2е - = 2Ќ+ + 0.5ќ2. —уммарна€ реакци€: FeSO4 + Ќ2ќ = Fe + Ќ2SO4 + 0.5ќ2 | |
| ƒано t = 5 часов = 18000 сек. ¬т = 70%, I = 20 ј. m(Zn) =? | m(Fe) = 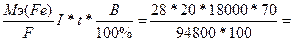 73 грамма 73 грамма
|
754. Ёлектролиз CuSO4
| атод: Cu2+ + 2е = Cu, Aнод: Ќ2ќ Ц 2е - = 2Ќ+ + 0.5ќ2. —уммарна€ реакци€: CuSO4 + Ќ2ќ = Cu + Ќ2SO4 + 0.5ќ2 | |
| ƒано V(O2) = 5,64 л, ¬т = 100% m(Cu) =? | ћэ(Cu) = 64/2 = 32 г/моль
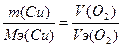 , следовательно, m(Cu) = 32*5.64/5.64 =32 грамма , следовательно, m(Cu) = 32*5.64/5.64 =32 грамма
|
755. Ёлектролиз ZnSO4
| атод: Zn2+ + 2е = Zn, Aнод: Ќ2ќ Ц 2е - = 2Ќ+ + 0.5ќ2. —уммарна€ реакци€: ZnSO4 + Ќ2ќ = Zn + Ќ2SO4 + 0.5ќ2 | |
| ƒано t = 6,7 часа = 24120 секунд V(O2) = 5,6 л, ¬т = 70% I =? | V(ќ2) = 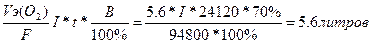 I=5.7A. I=5.7A.
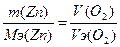 , следовательно, m(Zn) = 32.7*5.6/5.6 =32.7 грамма , следовательно, m(Zn) = 32.7*5.6/5.6 =32.7 грамма
|
756. Ёлектролиз K2SO4
| атод: 2Ќ2ќ + 2е = 2ќЌ- + Ќ2 анод: Ќ2ќ Ц 2е - = 2Ќ+ + 0.5ќ2. —уммарна€ реакци€: Ќ2ќ = Ќ2 + 0.5ќ2 | |
| ƒано I = 26.8 A t = 10часа = 36000 секунд ¬т = 100% | V(H2) = 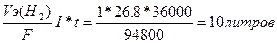 V(O2) = 1/2V(H2) = 5 литров
V(O2) = 1/2V(H2) = 5 литров
|
757. Ёлектролиз раствора ќЌ.
| атод: 2Ќ2ќ + 2е = 2ќЌ- + Ќ2 анод: 4ќЌ- - 4е = 2Ќ2ќ + ќ2 —уммарна€ реакци€ Ќ2ќ = Ќ2 + 1/2ќ2 | |
| ƒано I = 13.4 A t = 2часа = 7200 секунд ¬т = 100% | V(H2) = 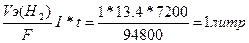 V(O2) = 1/2V(H2) = 0.5 литра
V(O2) = 1/2V(H2) = 0.5 литра
|
758. Ёлектролиз раствора Na2SO4
| атод: 2Ќ2ќ + 2е = 2ќЌ- + Ќ2, Na+ + OH- = NaOH анод: Ќ2ќ Ц 2е - = 2Ќ+ + 0.5ќ2, 2Ќ+ + SO42- = H2SO4 —уммарна€ реакци€: Ќ2ќ = Ќ2 + 0.5ќ2 | |
| ƒано V(O2) = 11.2 л m (H2SO4) =? | 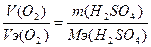 , m(H2SO4) = (11.2*49) / 5.6 = 98 г. , m(H2SO4) = (11.2*49) / 5.6 = 98 г.
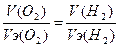 , V(H2) = (11.2*11.2) / 5.6 = 22.4 л. , V(H2) = (11.2*11.2) / 5.6 = 22.4 л.
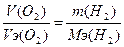 , m(H2) = (11.2*1) / 5.6 = 2 г. , m(H2) = (11.2*1) / 5.6 = 2 г.
|
759.
|
|
|
ƒано:
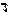 =300час =10,8*106сек.
I = 15 ј. =300час =10,8*106сек.
I = 15 ј.
| ѕоследовательность выделени€ веществ на электродах: атод: Cu2+ + 2e = Cu, Fe2+ + 2e = Fe, H2O + 2e = 2OH- + 1/2H2 јнод: 2Cl- - 2e = Cl2, H2O Ц 2e = 2H+ + 1/2O2 m = (Mэ/F)*Q, где ћэ Ц мол€рна€ масса эквивалента вещества, F=96500, Q Ц количество электричества. ћэ(Cu) = 64/2 = 32 г/моль, ћэ(Fe) = 56/2 = 28 г/моль. m(Cu) = n*M = 2*63.5 = 127 г. Ќа восстановление меди затрачено Q = m*F/Mэ = 127*96500/32 = 3.83*105 л. m(Fe) = n*M = 1*56 = 56 г. Ќа восстановление железа затрачено Q = m*F/Mэ = 56*96500/28 = 1.93*105 л. ¬сего было 10.8*106*15 = 1.62*108 л электричества. ѕосле восстановлени€ железа и меди осталось 1.62*108 Ц 1.93*105 Ц 3.83*105 = 1.61*108 л. ќставшеес€ электричество пошло на восстановление водорода, окисление воды и побочные реакции. ѕоскольку количество воды не дано,то количество водорода учесть невозможно. |
760.
ƒано:
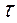 =1 час = 3600 сек.
I = 1 ј.
¬ = 2. - валентность
m = 2.219 г. =1 час = 3600 сек.
I = 1 ј.
¬ = 2. - валентность
m = 2.219 г.
| 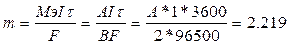 грамм.
ј = 118.9 а.е.м. Ёто олово - Sn. грамм.
ј = 118.9 а.е.м. Ёто олово - Sn.
|
761.
ƒано:
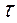 = 3 мин = 180 сек.
I = 10 ј.
m = 0.554 г.
V(Cl2) = 209 мл. = 3 мин = 180 сек.
I = 10 ј.
m = 0.554 г.
V(Cl2) = 209 мл.
| Ќайдем мол€рную массу металла:
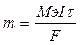 , следовательно, ћэ = , следовательно, ћэ = 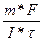 = (0.554*96487) / (10*180) = 29.7 г/моль, т.е. это олово: SnCl4 = (0.554*96487) / (10*180) = 29.7 г/моль, т.е. это олово: SnCl4
|
762.
ƒано:
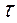 =30 мин=1800 сек.
I = 1,5 ј.
m = 1.071 г.
¬(ће) = 3
ће -? =30 мин=1800 сек.
I = 1,5 ј.
m = 1.071 г.
¬(ће) = 3
ће -?
| Ќайдем мол€рную массу металла:
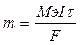 , следовательно, ћэ = , следовательно, ћэ = 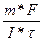 = (1.071*96487) / (1,5*1800) = 38.27 г/моль, ј(ће) = ¬*ћэ = 3*38.27 = 114.8 а.е.м., т.е. это индий. = (1.071*96487) / (1,5*1800) = 38.27 г/моль, ј(ће) = ¬*ћэ = 3*38.27 = 114.8 а.е.м., т.е. это индий.
|
763.
ƒано:
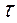 = 10 мин =600 сек.
I = 5 ј.
m = 1.517 г.
ћэ(Pt)-? = 10 мин =600 сек.
I = 5 ј.
m = 1.517 г.
ћэ(Pt)-?
| Ќайдем мол€рную массу металла:
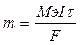 , следовательно, ћэ = , следовательно, ћэ = 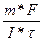 = (1.517*96487) / (5*600) = 48.8г/моль. = (1.517*96487) / (5*600) = 48.8г/моль.
|
764.
| ƒано: m(Ag) = 2.3 г. Q =?. | 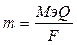 , следовательно, Q = , следовательно, Q = 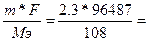 2055 л 2055 л
|
765.
ƒано:
I = 3 ј.
m = 1 моль.экв
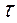 =?. =?.
| 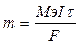 , следовательно, nэ= , следовательно, nэ= 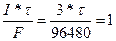
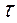 = 32160 секунд = 8.9 часов. = 32160 секунд = 8.9 часов.
|
766.
ƒано:
I = 1.5 ј.
h = 0.05 мм
l = 100 м
d = 50 мм = 5 см
¬т = 90 %
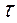 =?. =?.
| Ќайдем массу хромового покрыти€ (объем находим как разницу между объемами цилиндров): V(—r) = V1 Ц V2 = l*S1 Ц l*S2 = l(3.14*R12 Ц 3.14*R22) = 10*3.14(2.5052 Ц 2.52) = 0.7*10-4 см3. ѕлотность хрома p = 7.19 г/мл, следовательно масса m(Cr) = V*p = 0.7*10-4*7.19 = 5.65*10-4 г.
ћэ(Cr) = ћ/Ё = 52/3 = 17.33 г/моль.
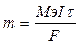 , следовательно, , следовательно, 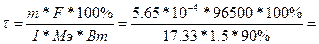 2.33 сек 2.33 сек
|
767.
ƒано:
I = 0.5 ј.
h = 8 мкм
S = 2 см2
m = 1 моль.экв
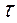 =?. =?.
| Ќайдем массу золотого покрыти€: V(Au) = h*S = 8*10-4*2 = 1.6*10-3 см3.
m(Au) = V*p = 1.6*10-3*19.3 = 0.031 г. ћэ(Au) = ћ/Ё = 197/3 = 65.67 г/моль.
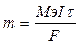 , следовательно, , следовательно, 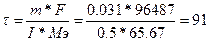 сек.
ƒл€ электролиза лучше вз€ть растворимую комплексную соль золота K[AuCl4] сек.
ƒл€ электролиза лучше вз€ть растворимую комплексную соль золота K[AuCl4]
|
768. Ќеобходимо загр€зненную медь растворить в азотной кислоте, а затем полученный раствор подвергнуть электролизу. ѕри этом медь восстановитс€ на катоде, цинк останетс€ в растворе, а серебро останетс€ на дне электролизера.
769. ƒл€ получени€ наиболее чистого нитрата кали€ из раствора данных солей необходимо провести электролиз. ѕри этом на аноде восстанавливаетс€ медь: Cu2+ +2e- = Cu0. Ќа катоде выдел€етс€ хлор: 2Cl- -2e- = Cl2. ¬ растворе остаетс€ нитрат кали€: K+ + NO3- = KNO3.
770. ѕри обычных услови€х гидролиз сульфата натри€ будет протекать в электролизере:
катод: H2O + 2e = 2OH- + 1/2H2, Na+ + OH- = NaOH
анод: H2O Ц 2e = 2H+ + 1/2O2, SO42- + 2H+ = H2SO4
771. ¬ зависимости от механизма, различают химическую и электрохимическую коррозии. ѕерва€ протекает, например, при взаимодействии металла с агрессивной газовой средой при высокой температуре. ¬тора€ Ц при ржавлении железного гвозд€ под воздействием воды и кислорода воздуха. ѕо форме коррозионных разрушений выдел€ют равномерную, местную, межкристальную, растрескивающуюс€ и компонентно-избирательную. Ћюбой вид коррозии ведет к разрушению металла, поэтому коррози€ всегда имеет отрицательное значение.
772. ’имическа€ коррози€ Ц окисление металла при отсутствии электролита, не сопровождающеес€ возникновением электрического тока в системе. Ёлектрохимическа€ коррози€ Ц разрушение металла в среде электролита в результате взаимодействи€ электрохимического характера. —корость коррозии определ€етс€ как масса разрушающегос€ металла в единицу времени.
773. ѕассивность металла Ц склонность последнего к образованию на своей поверхности труднорастворимых пленок (например, оксидных, гидроксидных), преп€тствующих протеканию реакции. “ак, при взаимодействии алюмини€ с кислородом воздуха, его поверхность покрываетс€ оксидом, вследствие чего алюминий не взаимодействует с водой ни с гор€чей ни с холодной. ѕоверхность железа пассивируетс€ концентрированной серной кислотой с образованием труднорастворимой соли, растворени€ железа в ней не наблюдаетс€. ѕоэтому концентрированную серную кислоту хран€т в железных бочках.
774. Ѕензин с примесью серы, сгора€, образует смесь —ќ2, H2O, SO2, SO3 и др. ¬ыхлопна€ труба при контакте с оксидом серы подвергаетс€ химической коррозии: Fe + SO3 + H2O = FeSO4 + H2
775. оррози€ никел€ в атмосфере —ќ €вл€етс€ химической коррозией. Ni + 4CO = [Ni(CO)4]
776. —ильнее всего корродирует треть€ бочка с раствором соды поскольку нар€ду с электрохимической протекает еще и химическа€ коррози€: т.к. это раствор, то есть вода, в атмосфере присутствует кислород, а в стали есть примеси, то образуетс€ гальвани-пара (-) Fe!H2O, O≠2! Cu (+). “.к. в растворе присутствуют ионы соли, то протекает реакци€ Ц химическа€ коррози€: Fe + 2Na2CO3 + 3H2O + 0.5O2 = Fe(HCO3)2 + 4NaOH
¬ меньшей степени корродирует бочка с водой, Ќо т.к. вода Ц электролит, а в атмосфере есть кислород, то образуетс€ аналогична€ предыдущей гальвани-пара и бочка подвергаетс€ электрохимической коррозии. Ќаиболее сильно коррози€ замедлена в случае бочки с керосином, поскольку керосин неэлектролит.
777.
| 4Au + 6H2O + 3O2 = 4Au(OH)3 ∆Go = 4∆Go(Au(OH)3) - 6∆Go(H2O) = 4*(-477.8) + 6*241.8 = - 460.4 кƒж/моль |
| 2Cu + 2H2O + O2 = 2Cu(OH)2 ∆Go = 2∆Go(Cu(OH)2) - 2∆Go(H2O) = 2*(-444.3) + 2*241.8 = - 405 кƒж/моль |
| 2Mg + 2H2O + 3O2 = 2Mg(OH)2 ∆Go = 2∆Go(Mg(OH)2) - 2∆Go(H2O) = 2*(-924.7) + 2*241.8 = - 1365.8 кƒж/моль |
ѕоскольку ∆Go всех трех реакций меньше 0, термодинамически все эти реакции возможны. ќднако на практике не все термодинамические реакции осуществимы при н.у., т.к. кроме термодинамических условий существуют еще и кинетические.
778. 4OH- - 4e- = 2H2O + O2 E = 0.40¬, 2Cl- - 2e- = Cl2 ≈ = 1.36¬. Ѕолее сильным окислителем будет сол€на€ кислота, так как ее потенциал наибольший. Fe + 2HCl = FeCl2 + H2. ≈сли не учитывать вли€ние растворенного кислорода и воды, то медленнее всего коррози€ железа будет протекать в растворе хлорида натри€.
779. огда трамвай проезжает по рельсам, под ними протекает блуждающий ток. Ётот ток вызывает коррозию наход€щихс€ вблизи металлических конструкций:
| (-)Fe(металлическа€ конструкци€)|H2O, O2|Fe(рельс)(+) (-): Fe(металлическа€ конструкци€) - 2e- = Fe2+ (+): 2H2O + O2 = 4OH- 2Fe + 2H2O + O2 = 2Fe(OH)2 |
780. Ѕольше пострадала та часть гвозд€, котора€ находилась в дереве.
| (-)Fe(внутри дерева)|H2O, O2|Fe(снаружи дерева)(+) (-): Fe(внутри дерева) - 2e- = Fe2+ (+): 2H2O + O2 = 4OH- 2Fe + 2H2O + O2 = 2Fe(OH)2 |
781. Fe + 2HCl = FeCl2 + H2, сжата€ пружина имеет большую внутреннюю энергию из-за деформации решетки. »онам железа требуетс€ меньше затрат энергии дл€ выхода из решетки в раствор. —ледовательно, сжата€ пружина растворитьс€ быстрее. ѕри замене нити на медную проволоку образуетс€ гальванический элемент и скорость растворени€ увеличиваетс€:
(-)Fe|HCl|Cu(+), (-): Fe -2e- = Fe2+ , (+) 2H+ +2e- = H2
782. ак при контакте меди с оловом, так и при контакте железа с оловом, образуетс€ гальванический элемент, в котором один из металлов становитс€ анодом и начинает корродировать. ќлово активнее меди, но менее активно, чем железо, поэтому в случае самовара корродирует олово, а в случае котелка Ц железо.
| (-)Fe(котелок)|H2O,O2|Sn(+) (-): Fe -2e- = Fe2+ (+): 2H2O + O2 = 4OH- 2Fe + 2H2O + O2 = 2Fe(OH)2 | (-)Sn|H2O,O2|Cu(самовар)(+) (-): Sn -2e- = Sn2+ (+): 2H2O + O2 = 4OH- 2Sn + 2H2O + O2 = 2Sn(OH)2 |
783. ќт коррозии лучше защищать будет то покрытие, которое при нарушении будет корродировать само, т.е. будет иметь меньший электродный потенциал, чем железо. ≈o(Fe2+/Fe) = -0.44 ¬, ≈o(Zn2+/Zn) = -0.76 ¬, ≈o(Sn2+/Sn) = -0.14 ¬, ≈o(Cr3+/Cr) = -0.74 ¬, ≈o(Cd2+/Cd) = -0.40 ¬, ≈o(Ni2+/Ni) = -0.25 ¬. Ќаиболее надежно будет защищать цинк, т.к. его потенциал наименьший. ѕо убыванию степени защиты: Zn>Cr>Cd>Ni>Sn.
784. јлюминий более активен, поэтому он будет корродировать в кислотной среде. (-) Al | H+ | Cu (+). E = Eo(Cu2+/Cu) - Eo(Al3+/Al) = 0.34 + 1.66 = 2 ¬. ∆Go = -z*F*E = -6*96480*2 = -1157.8 кƒж/моль
785. (-)Fe|Ќ2ќ, ќ2|C(+)
(Ц) Fe Ц 2e- = Fe2+;
(+) 2H2O + O2 = 4OH-
2Fe + 2H2O + O2 = 2Fe(OH)2
786. а) анод (-)Al|HCl|Fe (+) катод, Al Ц3e- =Al3+, 2H+ +2e- = H2, 2Al(OH)3 = 2AlCl3 + 3H2
б) анод (-)Al|KOH|Fe (+) катод, Al Ц3e- =Al3+, 2ќЌ- +4е- = 2Ќ2ќ + ќ2, Al + KOH + H2O = K3[Al(OH)6] + H2
787. ѕри коррозии железа и алюмини€ образуютс€ соответственно гидроксиды: Fe(OH)3, Al(OH)3, наличие которых на поверхности металла снижает скорость коррозии. ѕри растворении гидроксидов скорость коррозии увеличиваетс€. √идроксид железа хорошо раствор€етс€ в кислотах, а гидроксид алюмини€ в щелочах, поэтому коррози€ железа интенсивно протекает в кислой среде, а коррози€ алюмини€ Ц в щелочной.
788. ѕри помещении гвозд€ в раствор серной кислоты начинает протекать реакци€: Fe + H2SO4 = FeSO4 + H2. ѕри добавлении к раствору сульфата меди, медь, реагиру€ с железом, выдел€етс€ на поверхности гвозд€ (CuSO4 + Fe = Cu + FeSO4) и образуетс€ гальванический элемент, в котором медь выступает в роли инертного катода: (-)Fe|FeSO4||CuSO≠4|Cu(+). ¬ результате реакци€ железа с кислотой ускор€етс€ Ц увеличиваетс€ интенсивность выделени€ водорода. ѕри добавлении в раствор фосфорной кислоты катионы железа и меди св€зываютс€ в нерастворимые фосфаты, которые оседают на поверхность гвозд€ Ц реакци€ железа с кислотой прекращаетс€.
789. ѕри контакте меди с алюминием образуетс€ гальванический элемент, в котором алюминий €вл€етс€ анодом, а медь Ц катодом. Ќесмотр€ на то, что алюминий активнее меди и должен корродировать, этого не происходит, т.к. поверхность алюмини€ всегда покрыта прочной пленкой оксида, не позвол€ющей протекать коррозии. ѕоэтому в месте контакта корродирует медь.
790. Ќесмотр€ на то, что электродный потенциал алюмини€ меньше, чем цинка и алюминий должен легче коррозировать чем цинк, этого не происходит. Ќа воздухе алюминий покрываетс€ плотной пленкой оксида, котора€ предотвращает процесс коррозии. ѕоэтому цинк лучше защищает железо, чем алюминий. ѕри нарушении покрыти€ образуютс€ гальванические элементы:
| (-)Zn|H2O, O2|Fe (+) |
| (-)Fe|H2O, O2|Al (+) |
791. ÷инковое покрытие не выдерживает высокого термического расширени€, которое возникает при стрельбе из автомата. ¬оронение Ц нанесение на сталь оксидных пленок. ѕленки образуютс€ непосредственно на поверхности металла в результате высокотемпературного окислени€ или погружени€ стали в гор€чие концентрированные растворы щелочей, содержащих персульфаты, перхлораты, нитраты металлов.
792. ћеханизм действи€ значительного числа ингибиторов заключаетс€ в адсорбции ингибитора на корродирующей поверхности и последующем торможении катодных или анодных процессов анодным замедлител€м можно отнести нитрит натри€, дихромат натри€. катодным замедлител€м относ€тс€ органические вещества, содержащие азот, серу и кислород, например, уротропин, формальдегид, тиокрезол. «ащита от коррозии с помощью пассиваторов заключаетс€ в равномерном нанесении пассиватора на поверхность металла, дл€ отделени€ ее от агрессивной атмосферы. ѕримером такой защиты служит воронение, анодирование, покрытие лаками, красками, эмал€ми.
793. ¬ кислой среде потенциал водорода уменьшаетс€ и становитс€ меньше потенциала свинца, поэтому серную и сол€ную кислоты можно перекачивать по свинцовым трубопроводам. јзотна€ кислота окисл€ет свинец и ее нельз€ перекачивать по свинцовым трубам.
795. ƒл€ протекторной защиты металл выбираетс€ так, чтобы его стандартный электродный потенциал был меньше, чем потенциал металла, из которого состоит защищаема€ конструкци€. Ёлектрический ток в катодной защите подают дл€ того, чтобы защищаема€ конструкци€ стала катодным участком в образующейс€ цепи.
796. —тальной протектор удаетс€ сделать анодом при пропускании через всю буровую установку посто€нного электрического тока. ¬ результате защищаема€ конструкци€ становитс€ катодом и не корродирует. “ака€ защита называетс€ катодной.
797. орпус корабл€ €вл€етс€ одной цельной деталью, поэтому протектор защищает весь корпус. ¬ противоположность корпусу корабл€, корпус автомобил€ состоит из множества деталей, наход€щихс€ в плохом контакте друг с другом. ƒл€ катодной защиты автомобил€ потребовалось бы к каждому элементу корпуса прикрепить протектор, что не выгодно. ѕоэтому наиболее оптимальной защитой корпуса от коррозии €вл€ютс€ покрыти€: оцинковка, хромирование, эмали, лаки, мастики.
798. ”ротропин €вл€етс€ катодным замедлителем коррозии, который уменьшает скорость коррозии за счет снижени€ интенсивности катодного процесса или сокращени€ площади катодных участков. ¬заимодействие ржавчины с сол€ной кислотой:Fe(OH)3 + 3HCl = FeCl3 + 3H2O.
799. »з-за высокой цены золота, купола церквей покрывают более дешевыми материалами, сходными по цвету с золотом (SnS2, TiN). ќднако эти вещества не столь долговечны, как золото, поэтому покрытие приходитс€ часто обновл€ть.
800.
ƒано:
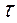 = 30 сек. I = 100ј.
D = 9 мм.
S = 20 см2
р = 7.6 г/см3
100 трамваев/сутки = 30 сек. I = 100ј.
D = 9 мм.
S = 20 см2
р = 7.6 г/см3
100 трамваев/сутки
| ј) Ќайдем объем железа V = D*S = 0.9*20 = 18 см3
Ѕ) ћасса железа равна m = V*p = 18*7.6 = 136.8 грамм
√) 1 трамвай разрушает 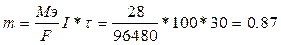 г. Fe
ƒ) “руба прослужит 136.8/(0.87*100) = 1.5 суток г. Fe
ƒ) “руба прослужит 136.8/(0.87*100) = 1.5 суток
|
831. чистое (ч), чистое дл€ анализа (ч.д.а.), химически чистое (х.ч.), особо чистое (ос.ч.).
832. ”величение степени чистоты вещества: техническое (т) Ц чистое (ч) Ц чистое дл€ анализа (ч.д.а.) Ц химически чистое (х.ч.) Ц особо чистое (ос.ч.) Ц спектрально чистое.
833. √азообразные вещества очищают от примесей следующими способами:фильтраци€ (от твердых примесей), адсорбци€ (при пропускании через газа адсорбент, поглощающий определенные примеси) и др.
834. ƒл€ очистки водорода от данных примесей потребуетс€ колонка заполненна€ порошком меди (очистка от кислорода), скл€нка со спиртом (поглощение H2S), колонка с ангидроном (осушение водорода).
835. ќт примесей воды углекислый газ можно очистить при помощи ангидрона, а взвесь MnO2 очистит газ от SO2.
836. јммиак от примесей —ќ2 можно отчистить аскаритом, а от воды при помощи ангидроном.
837. Ќаиболее полна€ осушка воздуха достигаетс€ во второй колонке, поскольку в ней конденсированна€ вода не может проникать дальше по трубе (как в первой и третьей колонке) и пар распределен равномерно по объему трубы, а не в какой-то ее части (как в третьей колонке).
838. ќт взвешенных примесей воду очищают фильтрацией, а от растворимых примесей воду можно очистить дистилл€цией, ионным обменом, электроосмосом и др.
839. –астворимые в воде вещества очищают от примесей перекристаллизацией: на первой стадии раствор€ют загр€зненное вещество: нерастворимые примеси отфильтровывают. Ќа второй стадии раствор можно пропустить через ионит дл€ отделени€ растворимых примесей, после чего воду выпаривают, получа€ чистое вещество.
840. ћеталлы от примесей очищают следующими способами и методами: зонна€ плавка, разложение летучих соединений, выжигание горючих примесей, электролиз и др.
841. ќчистка меди электролизом - достаточно дорогой процесс, ее используют, когда нужно получить особо чистый металл. Ёлектролитом служит сульфат меди CuSO4. Ќа аноде: Cu2+ +2e -= Cu0. Ќа катоде: H2O - 2e- = 2H+ + 1/2O2. —одержавшиес€ примеси - железо и цинк - остаютс€ в растворе, а серебро и золото осаждаютс€ на дно электролизера.
842. — помощью приведенной реакции возможно извлечь цирконий (и другие металлы) из руды, перевед€ его в газообразное соединение, которое, отделив от остальной смеси, можно разложить до чистого металла с очень высокой степенью чистоты. ƒанный метод очистки металлов называетс€ Ђразложение летучих соединенийї.
843. ѕолучаемое соединение называетс€ тетракарбонил никел€(0). — помощью приведенной реакции возможно извлечь никель (и другие металлы) из руды, перевед€ его в газообразное соединение, которое, отделив от остальной смеси, можно разложить до чистого металла с очень высокой степенью чистоты. ƒанный метод очистки металлов называетс€ Ђразложение летучих соединенийї.
844. ѕолучение особо чистых веществ методом зонной плавки заключаетс€ в следующем: смесь веществ помещают в колонну, которую нагревают до плавлени€ веществ. ѕлав€сь, вещества раздел€ютс€ на слои (зоны), причем существуют зоны с большим содержанием загр€знений, и зоны с чистым веществом, которое извлекают из колонны. ћетод основан на зависимости взаимной растворимости веществ от температуры.
845. јдсорбционные и ионообменные методы очистки веществ основаны на €влении адсорбции Ц накоплении одной фазы на поверхности другой фазы. Ёти методы используют, например, при очистке воды от ионов на катионитах и анионитах, очистке воздуха от газов активированным углем и т.п.
846. ƒл€ обнаружени€ элементов или ионов в качественном анализе используютс€ качественные реакции на группы ионов и на отдельные ионы. Ќапример на катионы третьей аналитической группы (Be2+, Al3+, Cr3+, Fe2+, Fe3+, Co2+, Zn2+, Ni2+ и др.) имеетс€ групповой реактив NaOH. ѕри действии этого группового реактива на данные ктаионы образуютс€ окрашенные в различные цвета гидроксиды: белые Be(OH)2, Ti(OH)4, Zr(OH)4, Mn(OH)2, Al()OH)3, Zn(OH)2, зеленые Cr(OH)3, Fe(OH)2, Ni(OH)2, бурокрасный Fe(OH)3, розовый Co(OH)2, черные Mn(OH)4, Ni(OH)3, Co(OH)3, желтый UO2(OH)2. —елективные реакции дл€ Fe3+:
а) 4Fe3+ + 3[Fe(CN)6]4+ = Fe4[Fe(CN)6]3 Ц темно-синий осадок
б) Fe3+ + 3SCN- = Fe(SCN)3 Ц кроваво-красный цвет
847.
| є аналитич. группы | катионы | √рупповой реагент |
| I (хлоридна€) | Hg2+ , Ag+ , Pb2+ | HCl |
| II (сульфатна€) | Ba2+, Ca2+, Sr2+ | H2SO4 |
| III(гидроксидна€) | Fe2+, Fe3+, Mn2+, Co2+ | NaOH |
| IV (амфолитна€) | Al3+, Zn2+, Cr3+, Sn2+ | NaOH (избыток) |
| V (аммиачна€) | Cu2+, Ni2+ | NH4OH |
| VI | Na+, K+, NH4+ | Ќет |
Ќа катионы VI аналитической группы нет группового реагента, т.к. трудно подобрать такой реагент, который осаждал все эти катионы в виде осадков.
848. јнионы дел€тс€ на 3 аналитических группы: анионы первой аналитической группы (SO42-, CO32-, PO43-, SiO32-, SO32-, S2O32-), групповой реактив - BaCl2. јнионы второй аналитической группы (Cl-, Br-, I-, S2- и др.), групповой реактив - AgNO3 . јнионы третьей группы: NO3-, NO2- и др, группового реагента нет.
849. а) атионы первой аналитической группы (—а2+, Sr2+, Ba2+, Ra2+). √рупповой реактив - NaOH, H2SO4. ѕри действии щелочи выпадают белые аморфные осадки гидроокисей. ѕри действии серной кислоты образуютс€ белые кристаллические осадки сульфатов. атионы второй аналитической группы (Be2+, Al3+, Fe2+, Ni2+ и др.). √рупповой реактив - NaOH. ѕри действии щелочи выпадают окрашенные осадки гидроокисей.
—а2+ + 2ќЌ- = —а(ќЌ)2
Sr2+ + H2SO4 = SrSO4 + 2H+
Ni2+ + 2OH- = Ni(OH)2 и т.п.
850. атионы третьей аналитической группы (Be2+, Al3+, Cr3+, Fe2+, Fe3+, Co2+, Zn2+, Ni2+ и др.): групповой реактив NaOH. ѕри действии группового реактива на данные ктаионы образуютс€ окрашенные в различные цвета гидроксиды: белые Be(OH)2, Ti(OH)4, Zr(OH)4, Mn(OH)2, Al()OH)3, Zn(OH)2, зеленые Cr(OH)3, Fe(OH)2, Ni(OH)2, бурокрасный Fe(OH)3, розовый Co(OH)2, черные Mn(OH)4, Ni(OH)3, Co(OH)3, желтый UO2(OH)2. ѕримеры:
| Fe3+ + 3OH- = Fe(OH)3 | Co3+ + 2OH- = Co(OH)2 | Zr4+ + 4OH- = Zr(OH)4 |
атионы второй аналитической группы (Hg2+, Cu2+, Bi3+, Cd2+, Pd2+, Sn2+, Sn4+, As3+ и др.): групповой реактив NaOH. ѕри действии группового реактива на данные ктаионы образуютс€ окрашенные в различные цвета гидроксиды: белые Sn(OH)2, Cd(OH)2, Bi(OH)3, краснобурые Au(OH)3, Pd(OH)2, сине-черный Ir(OH)3, Ir(OH)4 и др. ќбразующийс€ гидроксид ртути разлагаетс€ на желтую окись и воду. ѕримеры:
| Hg2+ + 2OH- = Hg(OH)2 = HgO + |







