621. »онное произведение воды - это тривиальное название константы диссоциации воды:  (при 220—). онстанта диссоциации по определению не зависит от концентрации веществ, вход€щих в реакцию - ионное произведение воды не зависит от количества добавленной щелочи или кислоты.
(при 220—). онстанта диссоциации по определению не зависит от концентрации веществ, вход€щих в реакцию - ионное произведение воды не зависит от количества добавленной щелочи или кислоты.
622. ¬одородный (рЌ) и гидроксильный (рќЌ) показатели вычисл€ютс€ через логарифмы концентраций соответствующих ионов: pH = -lg[H+], pOH = -lg[OH-].
| —реда | исла€ | ўелочна€ | Ќейтральна€ |
| [H+] | <10-7 | =10-7 | >10-7 |
| рЌ | <7 | =7 | >7 |
| рќЌ | >7 | =7 | <7 |
623. рЌ = -lg[H+], рЌ = 14 Ц рќЌ.
| [H+] | 10-4 | 10-11 | 4*10-6 | 1.78*10-7 | 4.92*10-3 |
| pЌ | 5.4 | 6.7 | 2.3 | ||
| pOH | 8.6 | 7.3 | 11.7 |
624. рЌ = 14 Ц рќЌ. рЌ = -lg[H+], [H+]*[OH-] = 10-14
| pЌ | 3.2 | 5.8 | 9.1 | 11.4 |
| [H+] | 6.3*10-4 | 1.6*10-6 | 7.9*10-10 | 4.0*10-12 |
| [OH-] | 1.6*10-11 | 6.3*10-9 | 1.3*10-5 | 2.5*10-3 |
625.
| ƒано: m(NaIOH) = 2 г. V(раствора)= 1л. pH, pOH -? | —м(NaOH) = m/(M*V) = 2/(40*1) = 0.05 моль/л рќЌ = -lg[OH-] = -lg0.05 = 1.3 pH = 14 Ц pOH = 14 Ц 1.3 = 12.7 |
626. —тепнь диссоциации азотной кислоты (a) составл€ет 82 %, следовательно [H+] = a*Cм = 0.82*1 = 0.82 моль/л. рЌ = -lg[H+] = -lg[0.82] = 0.086. рќЌ = 14 Ц рЌ = 13.914.
627. —Ќ3—ќќЌ = —Ќ3—ќќ - + Ќ+ . д = 1.754*10-5 , —(—Ќ3—ќќЌ) = 0.1 моль/л, [H+] = [CH3COO-] = х,  , х2 = 1.754*10-6 , х = [H+] = 1.32*10-3 моль/л.
, х2 = 1.754*10-6 , х = [H+] = 1.32*10-3 моль/л.
рЌ = -lg[H+], pH = -lg1.32*10-3 = 2.9, pOH = 14 Ц pH = 14 Ц 2.9 = 11.1
628.
| ƒано: V(NH3) = 2.24 л V(H2O) = 1 л. pH, pOH -? | оличество аммиака n(NH3) = V/Vм = 2.24/22.4 = 0.1 моль, следовательно, концентраци€ —м(NH3) = n/V = 0.1/1 = 0.1 моль/л.
Ќайдем концентрацию ќЌ-групп в растворе: степень диссоциации аммиака равна  =0.013. [OH-] = a*—м = 0.013*0.1 = 1.33*10-3 моль/л. рќЌ = -lg[OH-] = 2.9, pH = 14 Ц pOH = 11.1 =0.013. [OH-] = a*—м = 0.013*0.1 = 1.33*10-3 моль/л. рќЌ = -lg[OH-] = 2.9, pH = 14 Ц pOH = 11.1
|
629.
| ƒано: V(HNO3) = 1 л Cм(HNO3) = 1м m(NaOH) = 38 г. рH -? | HNO3 + NaOH = NaNO3 + H2O ќпределим количества обоих соединений: n(NaOH) = m/M =38/40 = 0.95 моль. n(HNO3) = Cм*V = 1 моль. ¬ растворе останетс€ 1 Ц 0.95 = 0.05 моль азотной кислоты, т.е. [H+] = 0.05 моль. pH = -lg[H+] = 1.3 |
630.
| »ндикатор | ќкраска | ¬ среде | рH, при котором »змен€етс€ окраска |
| ислой | ўелочной | ||
| Ћакмус | расный | —иний | 6,0-7,0 |
| ‘енолфталеин | Ѕесцветный | ћалиновый | 8,0-10,0 |
| ћетиловый оранжевый | ∆елтый | расный | 3,1-4,4 |
631. ѕ– рассчитываетс€, как произведение растворимостей ионов, где растворимость Ц концентраци€ вещества в его насыщенном растворе. «на€ растворимость, можно рассчитать ѕ–, и наоборот. “рифосфат серебра диссоциирует: Ag3PO4 = 3Ag+ + PO4 -3, тогда
S 3*3.8*10-5 3.8*10-5, n(AgNO3) = m/M = 6.5*10-3 / 170 = 3.8*10-5 моль/л.
“огда ѕ– будет S3 (Ag+)*S (PO4-3) = 1.3*10 Ц20 .
632. ѕ– рассчитываетс€, как произведение растворимостей ионов, где растворимость Ц концентраци€ вещества в его насыщенном растворе. «на€ растворимость, можно рассчитать ѕ–, и наоборот. »одид свинца диссоциирует PbI2 = Pb 2+ + 2I -, тогда
|
|
|
S 6,5*10-4 2*6,5*10-4,
“огда ѕ– будет S(Pb2+)*S2(I-) = 8,45*10-7
633. Ag2CO3 = 2Ag+ + CO32-
ѕ–(Ag2CO3) = 6.15*10-12. ѕ–(Ag2CO3) = [Ag+]2*[CO32-] = (2S)2*S = 4S3. S - растворимость.  = 1.15*10-4 моль/л. оэффициент растворимости выражаетс€ в граммах вещества, раствор€ющегос€ в 100 граммах воды. ¬ одном литре воды раствор€етс€ 1.15*10-4 моль соли, следовательно, в 100 граммах растворитс€ S = 1.15*10-4*ћ(Ag2CO3)/10 = 1.15*10-4*276/10 = 0.003 г/100г.
= 1.15*10-4 моль/л. оэффициент растворимости выражаетс€ в граммах вещества, раствор€ющегос€ в 100 граммах воды. ¬ одном литре воды раствор€етс€ 1.15*10-4 моль соли, следовательно, в 100 граммах растворитс€ S = 1.15*10-4*ћ(Ag2CO3)/10 = 1.15*10-4*276/10 = 0.003 г/100г.
634. Zn(OH)2 = Zn2+ + 2OH-
ѕ–(Zn(OH)2) = 4.0*10-16. ѕ–(Zn(OH)2) = [Zn2+]*[OH-]2 = S*(2S)2 = 4S3. S - растворимость.  = 4.64*10-6 моль/л. [Zn2+] = S = 4.64*10-6 моль/л, [OH-]= 2S = 2*4.64*10-6 = 9.28*10-6 моль/л.
= 4.64*10-6 моль/л. [Zn2+] = S = 4.64*10-6 моль/л, [OH-]= 2S = 2*4.64*10-6 = 9.28*10-6 моль/л.
635.
| ƒано: ѕ–(FeS) = 3.7*10-19 m(FeS) = 1г. V(H2O) =? | Ќайдем растворимость (S)данной соли: ѕ–(FeS) = [Fe2+]*[S2-] = S2, следовательно, S =  = 6.08*10-10 моль/л, т.е. в одном литре воды растворитс€ 6.08*10-10 моль FeS. n(FeS) = m/M = 1/88 = 0.011 моль.
V(H2O) = n/S = 0.011/6.08*10-10 = 1.7*107 литров = 6.08*10-10 моль/л, т.е. в одном литре воды растворитс€ 6.08*10-10 моль FeS. n(FeS) = m/M = 1/88 = 0.011 моль.
V(H2O) = n/S = 0.011/6.08*10-10 = 1.7*107 литров
|
636.
| ƒано: ѕ–(AgBr)=6.3*10-13 m(AgBr) = 1г. V(H2O) =? | Ќайдем растворимость (S)данной соли: ѕ–(AgBr) = [Ag+]*[Br -] = S2, следовательно, S =  = 7.94*10-7 моль/л, т.е. в одном литре воды растворитс€ моль 7.94*10-7 AgBr. n(AgBr) = m/M = 1/188 =5.3*10-3 моль.
V(H2O) = n/S = 5.3*10-3 / 7.94*10-7 = 6.7*103 литров = 7.94*10-7 моль/л, т.е. в одном литре воды растворитс€ моль 7.94*10-7 AgBr. n(AgBr) = m/M = 1/188 =5.3*10-3 моль.
V(H2O) = n/S = 5.3*10-3 / 7.94*10-7 = 6.7*103 литров
|
637.
| ƒано: ѕ–(MgS) = 2*10-15 Cн(Mg(NO3)2) = 4*10-3 н Cн(Na2S) = 6*10-4 н | ѕ– = [Mg2+]*[S2-] ѕри смешивании двух растворов одинакового объема их концентраци€ уменьшаетс€ в два раза: [Mg2+] = ½Cн(Mg(NO3)2) = 1/4Cм(Mg(NO3)2) = 1/4*0.0004 = 0.0001 моль/л [S2-] = 1/2Cн(Na2S) = 1/4Cн(Na2S) = 1/4*6*10-4 = 1.5 *10-4 моль/л [Mg2+]*[S2-] = 0.0001*0.00015 = 1.5*10-8 < ѕ–, следовательно, осадок не выпадет. |
638.
| ƒано: Cм(Cd(NO3)2) = 10-3 м Cм(Na2CO3) = 0.1 м ѕ–(CdCO3) = 2.5*10-14 | ѕ– = [Cd2+]*[CO32-] ѕри смешивании двух растворов одинакового объема их концентраци€ уменьшаетс€ в два раза: [Cd2+] = Cм(Cd(NO3)2) = ½*0.001 = 0.0005 моль/л [CO32-] = Cм(Na2CO3) = ½*0.1 = 0.05 моль/л [Cd2+]*[CO32-] = 0.0005*0.05 = 2.5*10-5 >> ѕ–, следовательно, осадок выпадет. |
639.
| ƒано: ѕ–(BaCO3) = 7*10-9 ѕ–(PbCO3) = 1.5*10-13 [Ba2+] / [Pb2+] =? | XCO3 = X2+ + CO32-, ѕ– = [X2+]*[CO32-]
 ; ;  , [CO32-]1 = [CO32-]2
[Ba2+]/[Pb2+] = ѕ–(BaCO3)/ѕ–(PbCO3) = 7*10-9/1.5*10-13 = 4.6*104 , [CO32-]1 = [CO32-]2
[Ba2+]/[Pb2+] = ѕ–(BaCO3)/ѕ–(PbCO3) = 7*10-9/1.5*10-13 = 4.6*104
|
640.
| ƒано: ѕ–(BaCrO4) = 2.3*10-10 ѕ–(SrCrO4) = 3.6*10-5 ≠ [Ba2+] = 5*10-4 моль/л [Sr2+] = 5*10-1 моль/л | XCO3 = X2+ + CO32- ѕ– = [X2+]*[CrO42-], [CrO42-] = ѕ–/[X2+] Ќайдем концентрацию CrO42-- ионов, необходимую дл€ выпадени€ осадков: а) [CrO42-] = ѕ–(BaCrO4)/[Ba2+] = 2.3*10-10/5*10-4 = 4.6*10-7 моль/л б) [CrO42-] = ѕ–(SrCrO4)/[Sr2+] = 3.6*10-5/5*10-1 = 7.2*10-5 моль/л ѕоскольку дл€ выпадени€ осадка SrCrO4 требуетс€ меньша€ концентраци€ CrO42-, то он выпадет первым |
641.
| ј) Na2S + 2HCl = 2NaCl + H2S S2- + 2H+ = H2S Ц бесцветный газ | ¬) Sn(OH)4 + 2H2SO4 = Sn(SO4)2 + 4H2O Sn(OH)4 + 4H+ = Sn2+ + 4H2O |
| Ѕ) Pb(NO3)2 + 2NaI = PbI2 + 2NaNO3 Pb2+ + 2I- = PbI2 Ц желтый осадок | √) Sn(OH)4 + 2KOH = K2[Sn(OH)6] Sn(OH)4 + 2OH- = [Sn(OH)6]2- |
642.
|
|
|
| ј) Ba(OH)2 + 2HCl = BaCl2 + 2H2O 2OH- + 2H+ = 2H2O | ¬) Al(OH)3 + 3HNO3 = Al(NO3)3 + 3 H2O 3OH- + 3H+ = 3H2O |
| Ѕ)NaHSO4 + NaOH = Na2SO4 + H2O HSO4- + 2OH- = SO42- + H2O | √) Al(OH)3 + 3KOH = K3[Al(OH)6] Al(OH)3 + 3OH- = [Al(OH)6]3- |
643.
| a) CaCO3 + 2HCl = CaCl2 + H2O + CO2 Ca2+ + 2H+ = H2O + CO2 | Ѕ) Na2S + FeSO4 = FeS + Na2SO4 S2- + Fe2+ = FeS |
| B) Zn(OH)2 + 2NaOH = Na2[Zn(OH)4] Zn(OH)2 + 2OH- = [Zn(OH)4]2- | √) Zn(OH)2 + 2HCl = ZnCl2 + 2H2O 2OH- + 2H+ = 2H2O |
644.
| Hg2+ + S2- = HgS | CO32- + 2H+ = H2O + CO2 |
| HgCl2 + K2S = HgS + 2KCl Hg(NO3)2 + H2S = HgS + 2HNO3 | Na2CO3 + 2HCl = 2NaCl + H2O + CO2 (NH4)2CO3 + 2HNO3 = 2NH4NO3 + H2O + CO2 |
645.
| Ca2+ + SiO32- = CaSiO3 | Al(OH)3 + 3OH- = [Al(OH)6]3- |
| CaCl2 + Na2SiO3 = CaSiO3 + 2NaCl Ca(NO3)2 + K2SiO3 = CaSiO3 + 2KNO3 | Al(OH)3 + 3NaOH = Na3[Al(OH)6] Al(OH)3 + 3KOH = K3[Al(OH)6] |
646.
| 2NO2- + 2H+ = NO + NO2 + H2O | Ag+ + Br- = AgBr |
| 2KNO2 + 2HCl = NO + NO2 + 2KCl + H2O 2NH4NO2 + 2HCN = NO + NO2 + 2NH4Cl + H2O | AgNO3 + KBr = AgBr + KNO3 AgNO3 + NaBr = AgBr + NaNO3 |
647. –еакци€ протекает в сторону более слабого электролита, т.е. в сторону образовани€ вещества с наименьшей ѕ–, д, н
| а) 2AgCl + Na2CrO4 = Ag2CrO4 + 2NaCl, ѕ–(Ag2CrO4) = 1.1*10-12< ѕ–(AgCl) = 1.73*10-10, следовательно реакци€ протекает в сторону образовани€ Ag2CrO4 |
| б) H2S + 2KNO2 = 2HNO2 + K2S, д(HNO2) = 6.9*10-4> д(H2S) = 1.0*10-7, следовательно реакци€ протекает в сторону образовани€ сероводорода. |
| в) [HgBr4]2- + 4Cl- = [HgCl4]2- + 4Br -, н([HgCl4]2 -)=8.5*10-16 > н([[HgBr4] ]2-) = 2.0*10-22, следовательно реакци€ протекает в сторону образовани€ [HgBr4]2- |
648. –еакци€ протекает в сторону более слабого электролита, т.е. в сторону образовани€ вещества с наименьшей ѕ–, д, н
| а) Ba3(PO4)2 + 3Na2SO4 = BaSO4 + 2Na3PO4, ѕ–(Ba3(PO4)2) = 6*10-39 < ѕ–(BaSO4) = 1.1*10-10, следовательно реакци€ протекает в сторону образовани€ фосфата бари€. |
| б) NH4OH + HCl = NH4Cl + H2O, д(NH4OH) = 7.5*10-14 > д(H2O) = 1*10-14, следовательно реакци€ протекает в сторону образовани€ воды. |
| в) [Cu(Cl)4]2- + 4CN- = [Cu(CN)4]2- + 4Cl- н([Cu(Cl)4]2-)=6.3*10-6> н([Cu(CN)4]2-) = 5*10-28, следовательно реакци€ протекает в сторону образовани€ [Cu(Cl)4]2- |
649. –еакци€ протекает в сторону более слабого электролита, т.е. в сторону образовани€ вещества с наименьшей ѕ–, д, н
| а) Cd(OH)2 + Na2CO3 = CdCO3 + 2NaOH ѕ–(Cd(OH)2) = 2.2*10-14 < ѕ–(CdCO3) = 1.0*10-12, следовательно реакци€ протекает в сторону образовани€ гидроксида кадми€ |
| б) CH3COOH + NaCN = CH3COONa + HCN д(CH3COOH) = 1.74*10-5 > д(HCN) = 5*10-10, следовательно реакци€ протекает в сторону образовани€ HCN |
| в) [Zn(OH)4]2- + 4CN- = [Zn(CN)4]2- + 4OH- н([Zn(OH)4]2-) = 3.6*10-16 > н([Zn(CN)4]2-)=1.3*10-17, следовательно реакци€ протекает в сторону образовани€ [Zn(CN)4]2- |
650. –еакци€ протекает в сторону более слабого электролита, т.е. в сторону образовани€ вещества с наименьшей ѕ–, д, н
| а) CaSO4 + Na2CO3 = CaCO3 + Na2SO4 ѕ–(CaSO4) = 2.5*10-5 > ѕ–(CaCO3) = 3.8*10-9, следовательно реакци€ протекает в сторону образовани€ карбоната кальци€ |
| б) Fe(OH)3≠ + 3NH4Cl = FeCl3 + 3NH4OH ѕ–(Fe(OH)3≠) = 6.3*10-29 < д(NH4OH) = 1.8*10-5, следовательно реакци€ протекает в сторону образовани€ гидроксида железа |
| в) [Ni(NH3)4]2+ + 4CN- = [Ni(CN)4]2- + 4NH3 н([Ni(NH3)4]2+) = 1.12*10-8 < н([Ni(CN)4]2-) = 1.8*10-14, следовательно реакци€ протекает в сторону образовани€ [Ni(CN)4]2- |
√лава 4.5. √идролиз солей
651. √идролиз солей Ц процесс, обратный реакции нейтрализации, т.е. взаимодействие соли с водой, привод€щее к образованию малодиссоциирующих соединений. “ипы гидролиза: по катиону (Cu(NO3)2, AlCl3), по аниону (K2CO3, Na2SO3), по обоим ионам одновременно (Al2≠S3, Ni(CN)2).√идролиз по катиону и аниону протекают обычно только по первой ступени и €вл€ютс€ обратимыми, в то врем€ как гидролиз одновременно по катиону и анионы - необратима€ реакци€, т.к. она протекает до конца.
|
|
|
652. √идролиз солей Ц процесс, обратный реакции нейтрализации, т.е. взаимодействие соли с водой, привод€щее к образованию малодиссоциирующих соединений. ќбразование слабых электролитов Ц причина протекани€ гидролиза.
| KNO2 + H2O = HNO2 + KOH NO2- + H2O = HNO2 + OH- | √идролиз по аниону, среда раствора щелочна€ |
| CuCl2 + H2O = CuOHCl + HCl Cu2+ + H2O = CuOH+ + H+ | √идролиз по катиону, среда раствора кислотна€ |
| Na3AsO4 + H2O = Na2HAsO4 + NaOH AsO4-3 + H2O = HAsO4-2 + OH- | √идролиз по аниону, среда раствора щелочна€ |
| Al2S3 + 6H2O = 2Al(OH)3 + 3H2S 2Al3+ + 3S2- + 6H2O = 2Al(OH)3 + 3H2S | √идролиз по катиону и аниону, среда практически нейтральна€ |
653. √идролиз солей Ц процесс, обратный реакции нейтрализации, т.е. взаимодействие соли с водой, привод€щее к образованию малодиссоциирующих соединений.
| K2CO3 + H2O = KHCO3 + KOH CO32- + H2O = HNO2 + OH- | √идролиз по аниону, среда раствора щелочна€ |
| NaAlO2 + H2O = NaOH + Al(OH)3 AlO2- + H2O = HAlO2 + OH- | √идролиз по аниону, среда раствора щелочна€ |
| (NH4)2SO4 + H2O = 2NH4OH + H2SO4 NH4+ + H2O = NH4OH + H+ | √идролиз по катиону, среда раствора кислотна€ |
| Cr2S3 + 6H2O = 2Cr(OH)3 + 3H2S 2Cr3+ + 3S2- + 6H2O = 2Cr(OH)3 + 3H2S | √идролиз по катиону и аниону, среда практически нейтральна€ |
654. √идролиз солей Ц процесс, обратный реакции нейтрализации, т.е. взаимодействие соли с водой, привод€щее к образованию малодиссоциирующих соединений.
| NH4CN + H2O = HCN + NH4OH NH4+ + CN - + H2O = HCN + NH4OH | √идролиз по катиону и аниону, среда практически нейтральна€ |
| K3PO4 + H2O = K2HPO4 + KOH PO4-3 + H2O = HPO4-2 + OH- | √идролиз по аниону, среда раствора щелочна€ |
| Pb(NO3)2 + H2O = Pb(OH)NO3 + HNO3 Pb+2 + H2O = PbOH+ + H+ | √идролиз по катиону, среда раствора кислотна€ |
| Fe2S3 + 6H2O = 2Cr(OH)3 + 3H2S 2Fe + + 3S2- + 6H2O = 2Fe(OH)3 + 3H2S | √идролиз по катиону и аниону, среда практически нейтральна€ |
655. √идролиз солей Ц процесс, обратный реакции нейтрализации, т.е. взаимодействие соли с водой, привод€щее к образованию малодиссоциирующих соединений.
| NH4NO3 + H2O = NH4OH + HNO3 NH4+ + H2O = NH4OH + H+ | √идролиз по катиону, среда раствора кислотна€ |
| Na2S + H2O = NaOH + H2S S-2 + H2O = H2S + OH- | √идролиз по аниону, среда раствора щелочна€ |
| FeCl3 + H2O = Fe(OH)Cl2 + HCl Fe+2 + H2O = FeOH+ + H+ | √идролиз по катиону, среда раствора кислотна€ |
| Al2(CO3)3 +6H2O = 2Al(OH)3 + 3H2CO3 2Al3++3CO32+6H2O=2Al(OH)3+3H2CO3 | √идролиз по катиону и аниону, среда практически нейтральна€ |
656. √идролиз солей Ц процесс, обратный реакции нейтрализации, т.е. взаимодействие соли с водой, привод€щее к образованию малодиссоциирующих соединений.
| CH3COOK + H2O = CH3COOH + KOH CH3COO - + H2O = CH3COOH + OH - | √идролиз по аниону, среда раствора щелочна€ |
| Ba(CN)2 + 2H2O = Ba(OH)2+ 2HCN 2CN - + 2H2O = 2HCN + 2OH- | √идролиз по аниону, среда раствора щелочна€ |
| 2ZnSO4 + 2H2O = (ZnOH)2SO4 + H2SO4 2Zn+2 + 2H2O = 2ZnOH+ + 2H+ | √идролиз по катиону, среда раствора кислотна€ |
| Al(NO2)3 +6H2O = Al(OH)3 + 3HNO2 Al3++3NO2-+6H2O=Al(OH)3+ 3HNO2 | √идролиз по катиону и аниону, среда практически нейтральна€ |
657. — позиции химической термодинамики, гидролиз Ц процесс, обратный реакции нейтрализации, т.е. взаимодействие соли с водой, привод€щее к образованию малодиссоциирующих соединений. „ем слабее образующийс€ электролит, тем полнее гидролизуетс€ соль. ј) »з солей KF и KNO2 полнее гидролизуетс€ перва€ соль, т.к. константа диссоциации HF (6.2*10-4) меньше константы диссоциации HNO2 (6.9*10-4). Ѕ) —оль CH3COONH4 гидролизуетс€ полнее, т.к. она представлена двум€ слабыми электролитами (CH3COOЌ и NH4ќЌ) и гидролизуетс€ необратимо, в то врем€ как соль CH3COONа представлена анионом слабой кислоты и катионом сильного основани€, т.е. гидролизуетс€ только по аниону.
|
|
|
| KNO2 + H2O = KOH + HNO2 NO2- + H2O = HNO2 + OH- среда щелочна€ | KF + H2O = KOH + HF F- + H2O = HF + OH- среда щелочна€ |
| CH3COONa + H2O = CH3COOH + NaOH CH3COO- + H2O = CH3COOH + OH- щелочна€ | CH3COONH4 + H2O = CH3COOH + NH4OH нейтр- CH3COONH4 + H2O = CH3COOH + NH4OH- альна€ |
658. — позиции химической термодинамики, гидролиз Ц процесс, обратный реакции нейтрализации, т.е. взаимодействие соли с водой, привод€щее к образованию малодиссоциирующих соединений. „ем слабее образующийс€ электролит, тем полнее гидролизуетс€ соль. ј) »з солей KCN и KClO полнее гидролизуетс€ перва€ соль, т.к. константа диссоциации HCN (5*10-10) меньше константы диссоциации HClO (3*10-8). Ѕ) —оль BeCl2 гидролизуетс€ полнее, чем MgCl2 т.к. Be(OH)2 Ц более слабое основание, чем Mg(OH)2
| KCN + H2O = KOH + HCN CN- + H2O = HCN + OH- среда щелочна€ | KClO + H2O = KOH + HClO ClO- + H2O = HClO + OH- среда щелочна€ |
| MgCl2 + H2O = Mg(OH)Cl + HCl Mg2+ + H2O = MgOH+ + H+ среда кислотна€ | BeCl2 + H2O = Be(OH)Cl + HCl Be2+ + H2O = BeOH+ + H+ среда кислотна€ |
659. — позиции строени€ вещества, гидролиз Ц процесс, обратный реакции нейтрализации, т.е. взаимодействие соли с пол€рными молекулами воды. ƒиполи воды окружают молекулы соли, что приводит к пол€ризации и диссоциации молекул соли. „ем больше пол€ризующее действие катиона и пол€ризуемость аниона, тем сильнее гидролизуетс€ соль. “.к. пол€ризующее действие и пол€ризуемость сильно завис€т от радиуса и зар€да иона, то соль гидролизуетс€ тем сильнее, чем больше зар€д и меньше радиус катиона и меньше зар€д и больше радиус аниона. ј) ѕолнее гидролизуетс€ FeCl3, а не FeCl2 т.к. в первой соли зар€д катиона железа выше. Ѕ) Na2CO3 гидролизуетс€ менее полно, чем Na2SiO3, т.к. размеры аниона SiO32- больше, чем аниона CO32-.
| FeCl2 + H2O = Fe(OH)Cl + HCl Fe2+ + H2O = FeOH+ + H+ среда кислотна€ | FeCl3 + H2O = Fe(OH)Cl2 + HCl Fe3+ + H2O = FeOH+2 + H+ среда кислотна€ |
| Na2—O3 + H2O = NaHCO3 + NaOH CO32- + H2O = HCO3- + OH- cреда щелочна€ | Na2SiO3 + H2O = NaHSiO3 + NaOH SiO32- + H2O = HSiO3- + OH- cреда щелочна€ |
660. √идролиз Ц процесс, обратный реакции нейтрализации, т.е. взаимодействие соли с пол€рными молекулами воды. ƒиполи воды окружают молекулы соли, что приводит к пол€ризации и диссоциации молекул соли. „ем больше пол€ризующее действие катиона и пол€ризуемость аниона, тем сильнее гидролизуетс€ соль. “.е. гидролиз протекает за счет разрыва ионных св€зей соли и образовани€ св€зей между ионами соли и дипол€ми воды.
| KNO2 + H2O = KOH + HNO2 NO2- + H2O = HNO2 + OH- | √идролиз по аниону, среда раствора щелочна€ |
| Na2—O3 + H2O = NaHCO3 + NaOH CO32- + H2O = HCO3- + OH- | √идролиз по аниону, среда раствора щелочна€ |
| Fe(NO3)3 + H2O = Fe(OH)(NO3)2 + HNO3 Fe3+ + H2O = FeOH2+ + H+ | √идролиз по катиону, среда кислотна€ |
661. —тупенчатый гидролиз наблюдаетс€, когда соль образована многоосновными кислотами или многокислотными основани€ми. „исло ступеней определ€етс€ степенью основности кислоты (основани€). —тепень гидролиза, как правило,от первой ступени к последней уменьшаетс€, т.к. константы диссоциации кислот и оснований падают, например, Ќ3–ќ4: а1>Ka2>Ka3. ¬ две ступени идет гидролиз FeCl2 + H2O = FeOHCl + HCl (1), FeOHCl + H2O = Fe(OH)2 + HCl
¬ три ступени Na3PO4 + H2O = Na2HPO4 + NaOH (1)
Na2HPO4 + H2O = NaH2PO4 + NaOH (2)
NaH2PO4 + H2O = H3PO4 + NaOH (3)
662. онстанта гидролиза солей многоосновных кислот или многокислотных оснований обычно велика только дл€ первой ступени, а дл€ второй невысока. ѕоэтому гидролиз таких солей проходит только в две ступени.
√идролиз в две ступени: K2S + H2O = KHS + KOH, KHS + H2O = H2S + KOH
√идролиз в три ступени: AlCl3 + H2O = Al(OH)Cl2 + HCl, Al(OH)Cl2 + H2O = Al(OH)2Cl + HCl, Al(OH)2Cl + H2O = Al(OH)3 + HCl
663. онстанта гидролиза Ц это константа равновеси€ реакции гидролиза, т.е. реакции взаимодействи€ соли с водой. онстанта гидролиза тем больше, чем более слабые электролиты образуютс€ при гидролизе, чем выше температура. ќт концентрации раствора константа гидролиза не зависит.
| NH4NO3 + H2O = NH4OH + HNO3 | г = 
|
| KNO2 + H2O = KOH + HNO2 | г = 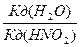
|
|
|
|
664.
| KClO + H2O = KOH + HClO ClO- + H2O = HClO + OH- | г = 
|
| K2S + H2O = KHS + KOH S2- + H2O = HS- + OH- | г = 
|
| AlCl3 + H2O = HCl + AlOHCl2 Al3+ + H2O = AlOH2+ + H+ | г = 
|
665. —тепень гидролиза Ц это отношение количества диссоциировавших молекул к количеству недиссоциированных молекул.  . ѕри увеличении концентрации раствора и уменьшении температуры степень гидролиза уменьшаетс€. ¬ведение в раствор щелочи или кислоты вли€ет на степень гидролиза по-разному. ≈сли соль гидролизуетс€ по катиону, то степень гидролиза усиливаетс€ при введении щелочи, а уменьшаетс€ при введении кислоты. ≈сли соль гидролизуетс€ по аниону, то степень гидролиза усиливаетс€ при введении кислоты, а уменьшаетс€ при введении щелочи.
. ѕри увеличении концентрации раствора и уменьшении температуры степень гидролиза уменьшаетс€. ¬ведение в раствор щелочи или кислоты вли€ет на степень гидролиза по-разному. ≈сли соль гидролизуетс€ по катиону, то степень гидролиза усиливаетс€ при введении щелочи, а уменьшаетс€ при введении кислоты. ≈сли соль гидролизуетс€ по аниону, то степень гидролиза усиливаетс€ при введении кислоты, а уменьшаетс€ при введении щелочи.
666. —тепень гидролиза соли тем выше, чем слабее образующиес€ электролиты.
| —оль | д | ѕо возрастанию степени гидролиза | ”равнени€ гидролиза |
| NaNO2 | ЌNO2 Ц 6.9*10-4 | NaNO2 + H2O = NaOH + HNO2 NO2- + H2O = HNO2 + OH- | |
| NaCN | HCN Ц 5.0*10-10 | NaCN + H2O = NaOH + HCN CN- + H2O = HCN + OH- | |
| NaClO | HClO Ц 3*10-8 | NaClO + H2O = NaOH + HClO ClO- + H2O = HClO + OH- | |
| NaClO2 | HClO2 Ц 1.1*10-2 | NaClO2 + H2O = NaOH + HClO2 ClO2- + H2O = HClO2 + OH- |
667. ѕри уменьшении концентрации соли в растворе и при увеличении температуры степень гидролиза возрастает. ѕри разбавлении растворов солей хлорида сурьмы и нитрата висмута степень гидролиза этих солей увеличиваетс€ Ц гидролиз протекает до конца и выпадают осадки соответствующих гидроксидов: SbCl3 + 3H2O = Sb(OH)3 + 3HCl, Bi(NO3)3 + 3H2O = Bi(OH)3 + 3HNO3.
668. ѕри увеличении температуры степень гидролиза солей возрастает. ѕри нагревании раствора ацетата натри€ степень гидролиза увеличиваетс€: CH3COONa + H2O = CH3COOH + NaOH. ¬ растворе растет концентраци€ ќЌ- групп, в результате фенолфталеин обесцвечиваетс€. ѕри охлаждении равновесие гидролиза соли смещаетс€ влево: раствор индикатора вновь приобретает малиновую окраску.
669. ѕри уменьшении концентрации соли в растворе и увеличении температуры степень гидролиза возрастает. ѕри добавлении хлорида железа по капл€м в кип€щую воду, гидролиз соли увеличиваетс€ настолько, что протекает до конца: FeCl3 + 3H2O = Fe(OH)3 + 3HCl
670. √идролиз уменьшает введение ионов одноименных ионам, образующимс€ при гидролизе. ≈сли соль гидролизуетс€ по катиону: FeCl3 + H2O = FeOH—l2 + HCl, то уменьшать гидролиз будут Ќ+. ≈сли соль гидролизуетс€ по аниону: NaClO2 + H2O = NaOH + HClO2, то уменьшать гидролиз будут ќЌ- анионы.
671. ѕри приготовлении растворов солей олова, сурьмы и висмута необходимо добавление кислоты дл€ подавлени€ гидролиза этих солей. ѕри отсутствии избытка кислоты соли гидролизуютс€ до образовани€ гидроксидов, например, SbCl3 + 3H2O = Sb(OH)3 + 3HCl, Bi(NO3)3 + 3H2O = Bi(OH)3 + 3HNO3.
672. ѕри приготовлении растворов алюминатов и хромитов необходимо добавление щелочи дл€ подавлени€ гидролиза этих солей. ѕри отсутствии избытка щелочи соли гидролизуютс€ до образовани€ гидроксидов, например, NaAlO2 + 2H2O = NaOH + Al(OH)3.
673. ѕри совместном гидролизе солей сульфата хрома и сульфида натри€ гидролиз солей протекает до конца, т.к. образуютс€ одноименные ионы, усиливающие гидролиз второй соли:
| Na2S + H2O = NaHS + NaOH S2- + H2O = HS- + OH- |
| Cr2(SO4)3 + 2H2O = 2CrOHSO4 + H2SO4 Cr3+ + H2O = CrOH2+ + H+ |
| H+ + OH- = H2O |
| 3Na2S + Cr2(SO4)3 + 6H2O = 3Na2SO4 + 2Cr(OH)3 + 3H2S |
674. ≈сли при совместном гидролизе двух солей образуютс€ одноименные ионы, усиливающие каждый гидролиз второй соли, то гидролиз таких солей протекает до конца. Ёто возможно, если из двух солей одна гидролизуетс€ по катиону, а втора€ по аниону:
а) Al(NO3)3, ZnCl2 Ц обе соли гидролизуютс€ по катиону и не усиливают гидролиз друг друга
б) Al2(SO4)3 и Na2CO3 Ц перва€ соль гидролизуетс€ по катиону, втора€ Ц по аниону, следовательно гидролиз солей усиливаетс€:
| Al3+ + H2O = AlOH2+ + H+ | CO32Ц + H2O = HCO3Ц + OHЦ |
| H+ + OHЦ = H2O | |
| 3Na2CO3 + Al2(SO4)3 + 6H2O = 3Na2SO4 + 2Al(OH)3 + 3H2CO3 |
в) AlCl3 и FeCl3 Ц обе соли гидролизуютс€ по катиону и не усиливают гидролиз друг друга
г) K2S и Pb(NO3)2 Ц перва€ соль гидролизуетс€ по аниону, втора€ Ц по катиону, следовательно гидролиз солей усиливаетс€:
| Pb2+ + H2O = PbOH+ + H+ | S2Ц + H2O = HSЦ + OHЦ |
| H+ + OHЦ = H2O | |
| K2S + Pb(NO3)2 + 2H2O = 2KNO3 + Pb(OH)2 + H2S |
675.
| ƒано —м(—Ќ3—ќќNH4) = 0.1м г =?, степень гидролиза а=? | —Ќ3—ќќNH4 + Ќ2ќ = CH3COOH + NH4OH
 =3.2*10-5
«акон ќствальда: г = —м*а2/(1-а) или а ~ =3.2*10-5
«акон ќствальда: г = —м*а2/(1-а) или а ~  = 0.018 = 1.8%. “.к. степень гидролиза относительно небольша€, то в данном случае гидролиз нельз€ считать необратимым процессом. ѕоскольку константа диссоциации NH4OH примерно равна константе диссоциации CH3COOH, то среда раствора практически нейтральна€. = 0.018 = 1.8%. “.к. степень гидролиза относительно небольша€, то в данном случае гидролиз нельз€ считать необратимым процессом. ѕоскольку константа диссоциации NH4OH примерно равна константе диссоциации CH3COOH, то среда раствора практически нейтральна€.
|
676.
| ƒано —м(NH4NO2) = 0.1м г =?, степень гидролиза а=? | NH4NO2 + Ќ2ќ = HNO2 + NH4OH
 = 8.1*10-7
«акон ќствальда: г = —м*а2/(1-а) или а ~ = 8.1*10-7
«акон ќствальда: г = —м*а2/(1-а) или а ~  = 0.003 = 0.3%. “.к. степень гидролиза относительно небольша€, то в данном случае гидролиз нельз€ считать необратимым процессом. ѕоскольку константа диссоциации NH4OH больше константы диссоциации HNO2, то среда раствора щелочна€. = 0.003 = 0.3%. “.к. степень гидролиза относительно небольша€, то в данном случае гидролиз нельз€ считать необратимым процессом. ѕоскольку константа диссоциации NH4OH больше константы диссоциации HNO2, то среда раствора щелочна€.
|
677.
| ƒано —м1(NaNO2) = 1м —м2(NaNO2) = 10-3м г =?, степень гидролиза а=? PH -? | NaNO2 + Ќ2ќ = HNO2 + NaOH
 = 1.45*10-11
«акон ќствальда: г = —м*а2/(1-а) или а ~ = 1.45*10-11
«акон ќствальда: г = —м*а2/(1-а) или а ~  a) a1 =
a) a1 =  = 3.8*10-6 б) a2 = = 3.8*10-6 б) a2 =  = 1.2*10-4
— увеличением разбавлени€ степень гидролиза соли увеличиваетс€.
[H+] = a1*Cм1 = 3.8*10-6 моль/л.
рЌ = -lg[H+] = 5.4 = 1.2*10-4
— увеличением разбавлени€ степень гидролиза соли увеличиваетс€.
[H+] = a1*Cм1 = 3.8*10-6 моль/л.
рЌ = -lg[H+] = 5.4
|
678.
| ƒано —м(Na2—O3) = 1м г =?, степень гидролиза а =? pH -? | Na2—O3 + Ќ2ќ = NaHCO3 + NaOH
 = 2*10-4 = 2*10-4
| NaH—O3 + Ќ2ќ = H2CO3 + NaOH
 = 2*10-8 = 2*10-8
|
«акон ќствальда: г = —м*а2/(1-а) или а ~  a1 =
a1 =  = 1.4*10-2,Cм(NaHCO3) = a*Cм(H2CO3) = 0.014 моль/л
б) a2 = = 1.4*10-2,Cм(NaHCO3) = a*Cм(H2CO3) = 0.014 моль/л
б) a2 =  = 1.2*10-3. —тепень гидролиза соли по второй ступени небольша€ и вклад 2-й = 1.2*10-3. —тепень гидролиза соли по второй ступени небольша€ и вклад 2-й  = 2*10-8 ступени гидролиза в значение рЌ можно не учитывать.
[ќH-] = a1*Cм = 0.014 моль/л.
рќЌ = -lg[ќH-] = 1.8, следовательно рЌ = 14- рќЌ = 12.2 = 2*10-8 ступени гидролиза в значение рЌ можно не учитывать.
[ќH-] = a1*Cм = 0.014 моль/л.
рќЌ = -lg[ќH-] = 1.8, следовательно рЌ = 14- рќЌ = 12.2
|
679.
| ƒано —м(K2SO3) = 1м г =?, степень гидролиза а =? pH -? | K2SO3 + Ќ2ќ = KHSO3 + KOH
 = 1.16*10-7 = 1.16*10-7
| KHSO3 + Ќ2ќ = H2SO3 + KOH
 = 7.15*10-13 = 7.15*10-13
|
«акон ќствальда: г = —м*а2/(1-а) или а ~  a1 =
a1 =  = 3.4*10-4,Cм(KHSO3) = a*Cм(H2SO3) = 3.4*10-4 моль/л
б) a2 = = 3.4*10-4,Cм(KHSO3) = a*Cм(H2SO3) = 3.4*10-4 моль/л
б) a2 =  = 4.6*10-5. —тепень гидролиза соли по второй ступени небольша€ и вклад 2-й = 4.6*10-5. —тепень гидролиза соли по второй ступени небольша€ и вклад 2-й  = 7.15*10-13 ступени можно не учитывать.
[ќH-] = a1*Cм = 3.4*10-4 моль/л. рќЌ = -lg[ќH-] = 4.5, рЌ = 14- рќЌ = 9.5 = 7.15*10-13 ступени можно не учитывать.
[ќH-] = a1*Cм = 3.4*10-4 моль/л. рќЌ = -lg[ќH-] = 4.5, рЌ = 14- рќЌ = 9.5
|
680.
1) NO2 + Ќ2ќ = OH + HNO2
 = 1.15*10-11 = 1.15*10-11
| 2) NaNO2 + Ќ2ќ = NaOH + HNO2
 = 1.15*10-11 = 1.15*10-11
|
«акон ќствальда: г = —м*а2/(1-а) или а ~ 
a1 = a2 =  = 1.2*10-5,Cм(ЁNO3) = a*Cм(HNO3) = 1.2*10-5 *0.1 = 1.2*10-6 моль/л
= 1.2*10-5,Cм(ЁNO3) = a*Cм(HNO3) = 1.2*10-5 *0.1 = 1.2*10-6 моль/л
[Ќ+] = 1.2*10-6 моль/л. рЌ = -lg[H+] = 5.9.
ќдинаковый результат дл€ двух разных солей объ€сн€етс€ тем, что при гидролизе обе соли дают одну и туже кислоту - HNO2, константу диссоциации которой мы и подставл€ем в формулу дл€ расчета константы гидролиза.
683. ќкислительно-восстановительный потенциал характеризует окислительно-восстановительную активность вещества, стабильность степеней окислени€ веществ, возможные переходы между ними.
„ем меньше окислительно-восстановительный потенциал, тем сильнее восстановительные свойства, например: Li (E0 = -3.05 B), K (E0 = -2.93 B). „ем больше окислительно-восстановительный потенциал, тем сильнее окислительные свойства, например: —l/Cl2 (E0 = 1.36 B), O3 (E0 = 1.51 B).
685. ќкислительно-восстановительный потенциал €вл€етс€ термодинамическим параметром, т.к. определ€ет возможность протекани€ процесса. 
 ,
,  , где ≈ Ц электродвижуща€ сила, F Ц посто€нна€ ‘араде€, R Ц газова€ посто€нна€, “ - температура, z Ц количество электронов.
, где ≈ Ц электродвижуща€ сила, F Ц посто€нна€ ‘араде€, R Ц газова€ посто€нна€, “ - температура, z Ц количество электронов.
687. MnO4- + 8H+ + 5e- = Mn 2+ + 4H2O, ≈ = 1.51 ¬.
—м = 1 ћ, [H+] = 5ћ, [Mn2+] = 0.01M, “ = 298 .
≈ (MnO4- / Mn 2+) = ≈0 (MnO4- / Mn 2+) + (2.3*R*T / n*F) *ln (—м MnO4- *—м8H+) / —м Mn 2+
≈ (MnO4- / Mn 2+) = 1.51 + (2.3*8.31*298 / 5*96500) * ln (1*58 /0.01) = 1.72 ¬.
688. Cr2O7-2 + 14H+ + 6e- = 2Cr 3+ + 7H2O, ≈ = 1.33 ¬.
—м = 1 ћ, [H+] = 5ћ, [Cr3+] = 0.1M, “ = 298 .
≈ (Cr2O7-2 / Cr 3+) = ≈0 (Cr2O7-2/ Cr +3) + (2.3*R*T / n*F) *ln (—м Cr2O7-2 *—м14H+) / —м Cr 2+
≈ (Cr2O7-2 / Cr 3+) = 1.33 + (2.3*8.31*298 / 6*96500) * ln (1*514 /0.1) = 1.69 ¬.
689.
| ƒано W%(H2SO4) = 60% p = 1.50 г/мл T = 298 K E -? | SO42- + 8H+ + 6e = S + 4H2O E(SO42-/S) = Eo(SO42-/S) +  Ќайдем концентрацию серной кислоты: возьмем 1 литр раствора.
m(р-ра H2SO4) = V*p = 1000*1.50 = 1500 грамм.
m(H2SO4) = m(р-ра H2SO4)*W%/100% = 1500*60/100 = 900 грамм.
Cм = m/(M*V) = 900/(98*1) = 9.2 моль/л.
—м(H2SO4) = —м(SO42-) = 9.2, —м(Ќ+) = 2—м(H2SO4) = 18.4 моль/л.
E(SO42-/S) = 0.36 +
Ќайдем концентрацию серной кислоты: возьмем 1 литр раствора.
m(р-ра H2SO4) = V*p = 1000*1.50 = 1500 грамм.
m(H2SO4) = m(р-ра H2SO4)*W%/100% = 1500*60/100 = 900 грамм.
Cм = m/(M*V) = 900/(98*1) = 9.2 моль/л.
—м(H2SO4) = —м(SO42-) = 9.2, —м(Ќ+) = 2—м(H2SO4) = 18.4 моль/л.
E(SO42-/S) = 0.36 + 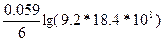 = 0.46 ¬ = 0.46 ¬
|
ѕолученный результат €вл€етс€ приблизительным, поскольку вычислени€ ведутс€ из предположени€, что серна€ кислота диссоциирует нацело.
690.
| ƒано W%(HNO3)=40% p = 1.25 г/мл T = 298 K E -? | NO3- + 4H+ + 3e = NO + 2H2O E(NO3-/NO) = Eo(NO3-/NO) +  Ќайдем концентрацию азотной кислоты: возьмем 1 литр раствора.
m(р-ра HNO3) = V*p = 1000*1.25 = 1250 г.
m(HNO3) = m(р-ра HNO3)*W%/100% = 1250*40/100 = 500 грамм.
Cм = m/(M*V) = 500/(63*1) = 7.94 моль/л.
—м(HNO3) = —м(NO3-) = —м(Ќ+) = 7.94 моль/л.
E(NO3-/NO) = 0.96 +
Ќайдем концентрацию азотной кислоты: возьмем 1 литр раствора.
m(р-ра HNO3) = V*p = 1000*1.25 = 1250 г.
m(HNO3) = m(р-ра HNO3)*W%/100% = 1250*40/100 = 500 грамм.
Cм = m/(M*V) = 500/(63*1) = 7.94 моль/л.
—м(HNO3) = —м(NO3-) = —м(Ќ+) = 7.94 моль/л.
E(NO3-/NO) = 0.96 +  = 1.048 ¬ = 1.048 ¬
|
ѕолученный результат €вл€етс€ приблизительным, поскольку вычислени€ ведутс€ из предположени€, что азотна€ кислота диссоциирует нацело. роме того невозможно учесть концентрацию выдел€ющегос€ NO, который вли€ет на положение равновеси€ данной реакции.
692. Cr2O72- + 14H+ + 6e - =2Cr3+ + 7H2O, j = 1.33 B.
CrO42- + 4H2O + 3e - =Cr3+ +8OH -, j = -0.13 B.
j(окислител€) > j(восстановител€), в соответствии с этим правилом, хром €вл€етс€ более сильным восстановителем в щелочной среде а более сильным окислителем Ц в кислой среде.
693. Ѕолее сильным восстановителем цинк €вл€етс€ в щелочной среде, поскольку в ней он имеет потенциал ≈0 (Zn2+ /Zn) = - 1.24 ¬, а в кислой ≈0 = -0.76 ¬.
694. ¬ кислой и нейтральной средах:Al 3+ + 3e - = Al, j = - 0.67 B.
¬ щелочной среде: Al + 3OH - - 3e - = Al(OЌ)3 , j = -2.30 B.
„ем меньше потенциал восстановител€, тем больше будет разница между ним и потенциалом окислител€, в соответствии с этим правилом, алюминий €вл€етс€ более сильным восстановителем в щелочной среде.
695. —огласно правилу, ≈(окислител€) должно быть > ≈(восстановител€). ѕерхлораты в качестве окислителей целесообразно использовать в кислой среде, т.к. максимальный потенциал ≈ = 1.58 ¬. хлораты используют в щелочной среде (≈ = 1.49 ¬). ’лориты Ц в кислой среде (≈ = 1.21 ¬). √ипохлориты - в кислой (≈ = 1.63 ¬).
696. Ёлектроны, наход€щиес€ в объеме металла при контакте его с раствором соли могут переходить в раствор или в металл Ц возникает зар€д. ќн называетс€ двойным электрическим слоем и зависит от потенциала металла, концентрации соли и природы металла.
697. Ёлектродные потенциалы металлов и их ионизационные потенциалы определ€ютс€ различными методами. »онизационные определ€ютс€ спектральными методами, а электродные Ц относительно стандартного водородного электрода, поэтому значени€ этих двух величин не совпадают.
698. ¬ р€ду напр€жений металлы расположены в пор€дке убывани€ их отрицательных и возрастани€ положительных потенциалов. –€д обладает свойствами:
- каждый из наход€щихс€ в р€ду металлов вытесн€ет из растворов их солей все металлы, следующие за ним. ѕоэтому кадмий будет вытесн€ть медь и серебро, но не вытеснит цинк и магний. ¬ свою очередь кадмий вытеснит любой металл, сто€щий слева от него, это может быть калий, магний, алюминий, железо и др.
- все металлы, сто€щие в р€ду напр€жений до водорода вытесн€ют его из растворов кислот.
- восстановительна€ способность металлов падает слева направо.
701. I (0.62 B) Е Br (1.09) Е Cl (1.36) Е F (2.87)
–€д обладает свойствами: чем правее стоит элемент, тем сильнее его окислительна€ способность.
702.
| HCl + O2 = Cl2 + 2H2O 2Cl-1 Ц 1e- = Cl2 (E = 1.36 ¬) O2 + 4H+ + 4e- = 2H2O (≈ = 1.23 ¬) —огласно правилу, ≈(окислител€) должно быть > ≈(восстановител€). “.е. окислителем в данной реакции €вл€етс€ O2 - реакци€ протекает в обратном направлении. |
| H3PO4 + HI = H3PO3 +I2 PO4 3- + 2H+ +2e- = PO3 3- + H2O, (E = -0.28 ¬) 2I -1 - 2eЦ = I2 (≈ = 0.62 ¬), ≈(окислител€) > ≈(восстановител€). “.е. окислителем в данной реакции €вл€етс€ H3PO4 - реакци€ протекает в обратном направлении. |
703.
| 2HCl + 2HNO3 = 2NO + Cl2 + 2H2O 2Cl-1 Ц 1e- = Cl2 (E = 1.36 ¬) NO3- + 4H+ + 3e- = NO + 2H2O (≈ = 0.96 ¬) —огласно правилу, ≈(окислител€) должно быть > ≈(восстановител€). “.е. окислителем в данной реакции €вл€етс€ HNO3 - реакци€ протекает в обратном направлении. |
| 2FeSO4 + 2I2 + H2SO4 = Fe2(SO4)3 +4HI Fe 2+ - 1e- = Fe 3+ (E = 0.77 ¬) I2 + 2eЦ = 2I -1 (≈ = 0.62 ¬) ≈(окислител€) > ≈(восстановител€). “.е. окислителем в данной реакции €вл€етс€ I2 - реакци€ протекает в обратном направлении. |
704.
| 2KMnO4 + 8KBr + 4H2O = 2MnO2 + 4Br2 + 8KOH MnO4- + 2H2O + 3e- = MnO2 + 4OH- , E = 1.23 ¬ 2Br- -2e- = Br2, ≈ = 1.09 ¬ —огласно правилу, ≈(окислител€) должно быть > ≈(восстановител€). “.е. окислителем в данной реакции €вл€етс€ KMnO4 - реакци€ протекает в пр€мом направлении. |
| 2KMnO4 +6Ag + H2O = 2MnO2 + 3Ag2O + 2KOH MnO4- + 2H2O + 3e- = MnO2 + 4OH- , E = 1.23 ¬ 2Ag + 2OH- Ц2e = Ag2ќ + H2O, ≈ = 0.79 ¬ ≈(окислител€) > ≈(восстановител€). “.е. окислителем в данной реакции €вл€етс€ KMnO4 - реакци€ протекает в пр€мом направлении. |
705.
| H≠3PO3 + SnCl2 + H2O = H≠3PO4 + Sn + 2HCl PO3 3- + H2O -2e- = PO4 3- + 2H+, E = -0.28 ¬ Sn2+ +2e- = Sn, ≈ = -0.14 ¬ —огласно правилу, ≈(окислител€) должно быть > ≈(восстановител€). “.е. окислителем в данной реакции €вл€етс€ SnCl2 - реакци€ протекает в пр€мом направлении. |
| SnCl2 + I2 + KCl = SnCl4 + KI Sn2+ -2e- = Sn4+, ≈ = 0.15 ¬, I2 +2e- = 2I-, ≈ = 0.62 ¬ ≈(окислител€) > ≈(восстановител€). “.е. окислителем в данной реакции €вл€етс€ иод - реакци€ протекает в пр€мом направлении. |
706.
| Se + 2H2SO4 + H2O = H2SeO3 + H2SO3 Se + 6OH-Ц 4e- = SeO32- + 3H2O, (E = -0.37¬) SO4-2 + H2O + 2e- = SO32- + 2OH-, (≈ = -0.93 ¬) —огласно правилу, ≈(окислител€) должно быть > ≈(восстановител€). “.е. окислителем в данной реакции €вл€етс€ H2SO4 - реакци€ протекает в обратном направлении. |
| Cu + Br2 + Na2SO4 = CuSO4 + NaBr Cu - 2e- = Cu+2 , (E = 0.337 ¬) Br2 +2eЦ = 2Br -, (≈ = 1.09 ¬), ≈(окислител€) > ≈(восстановител€). “.е. окислителем в данной реакции €вл€етс€ Br2 - реакци€ протекает в пр€мом направлении. |
707.
| Ag + 2HNO3 = AgNO3 + NO2 + H2O Ag Ц 1e- = Ag+ (E = 0.790¬) NO3- + 2H+ + 1e- = NO2 + H2O (≈ = 0.80 ¬) —огласно правилу, ≈(окислител€) должно быть > ≈(восстановител€). “.е. окислителем в данной реакции €вл€етс€ HNO3 - реакци€ протекает в пр€мом направлении. |
| Cl2 + H2O = HCl + HClO Cl2 + 2e- = 2ClЦ (E = 1.36 ¬) Cl2 + H2O Ц2eЦ = 2HClO + 2H+ (≈ = 1.63 ¬) ≈(окислител€) > ≈(восстановител€). “.е. окислителем в данной реакции €вл€етс€ Cl2 - реакци€ протекает в пр€мом направлении. |
708.
| Au + 6HNO3 = Au(NO3)3 + 3NO2 + 3H2O Au Ц 3e- = Au3+ E = 1.5 ¬ NO3- + 2H+ + 1e- = NO2 + H2O ≈ = 0.8 ¬ —огласно правилу, ≈(окислител€) должно быть > ≈(восстановител€). “.е. окислителем в данной реакции €вл€етс€ Au3+ - реакци€ протекает в обратном направлении. |
| PbSO4 + H2O2 = PbO2 + H2SO4 Pb2+ Ц 2e- = Pb4+ E = 1.69 ¬ H2O2 = 2H+ + O2- ≈ = 0.68 ¬ ≈(окислител€) > ≈(восстановител€). “.е. окислителем в данной реакции €вл€етс€ Pb4+ - реакци€ протекает в обратном направлении. |
709. а) HIO3 про€вл€ет окислительные свойства (≈ = 1.08 ¬), тогда как HCl Ц слабый восстановитель (≈ = 1.36 ¬). ѕоскольку ќ¬– может протекать при условии, что ≈(окислител€) > ≈(восстановител€), то данные вещества не будут взаимодействовать и можно на их основе приготовить раствор. б) K2Cr≠2O7 Ц сильный окислитель (≈ = 1.33 ¬), а KBr Ц слабый восстановитель (≈ = 1.09 ¬). ƒанные вещества могут взаимодействовать и раствор из них приготовить нельз€.
710. —огласно правилу, ≈(окислител€) должно быть > ≈(восстановител€).
а) ≈ (HNO3) = 0.80 B, ≈ (HBr) = 1.09 B. ѕотенциал окислител€ (азотной кислоты) меньше потенциала восстановител€, поэтому HNO3 не будет окисл€ть HBr, раствор можно приготовить.
б) по той же причине железо не восстановит хром до шестивалентного состо€ни€, такой раствор тоже можно приготовить.






