504.
| Ќазвание комплекса | омплекс | ќ | —ќ | „ | «ар€д |
| “етрахлороплатинат(II) аммони€ | (NH4)2[Pt(—l)4] | платина | +2 | -2 | |
| Ќитрат пентаамминроданокобальта(III) | [Co(NH3)5—NS](NO3)2 | кобальт | +3 | +2 | |
| √ексабромоплатинат(IV)тетрааквацинка(II) | [Zn(H2O)4][PtBr6] | ÷инк-платина | +2 +4 | +2 -2 |
505.
| Ќазвание комплекса | омплекс | ќ | —ќ | „ | «ар€д |
| √ексагидроксоалюминат кали€ | K3[Al(OH)6] | јлюминий | +3 | -3 | |
| √идросульфат пентаамминнитратокобальта(III) | [Co(NH3)5NO3](HSO4)2 | обальт | +3 | +2 | |
| √ексахлороплатинат(IV) гексаакважелеза(II) | [Fe(H2O)6][PtCl6] | железо-платина | +2 +4 | +2 -2 |
506.
| Ќазвание комплекса | омплекс | ќ | —ќ | „ | «ар€д |
| гексацианоферрат(III) натри€ | Na3[Fe(CN)6] | ∆елезо | +3 | -3 | |
| Ѕромид гексааминхрома(III) | [Cr(NH3)6]Br3 | ’ром | +3 | +3 | |
| √ексафтороманганат(IV) тетраакваникел€(II) | [Zn(H2O)4][MnF6] | ÷инк-марганец | +2 +4 | +2 -2 |
507.
| омплекс | Ќазвание комплекса | ќсобенности |
| [Cr(H2O)5Cl](NO3)2 | √ексааквахлорохром(III) нитрат | —оль, катионное, смешанное. |
| H[AuCl4] | ¬одорода тетрахлороаурат(III) | ислота, анионное, ацидокомплексное. |
| [Cu(NH3)4](OH)2 | “етраамминмедь(II) гидроксид | ќснование, катионное, амминокомплексное |
| [Fe(H2O)6]SO4 | √ексаакважелезо(II)сульфат | —оль, катионное, аквакомплекс |
508.
| омплекс | Ќазвание комплекса | ќсобенности |
| H2[SiF6] | √ексааквахлорохром(III) нитрат | ислота, анионное, ацидокомплексное |
| [Zn(NH3)4](OH)2 | ¬одорода тетрахлороаурат(III) | ќснование, катионное, амминокомплексное. |
| Na[Co(NH3)2(NO2)4] | “етраамминмедь(II) гидроксид | —оль, анионное, смешанное. |
| [Fe(H2O)6]2(SO4)3 | √ексаакважелезо(III)сульфат | —оль, катионное, аквакомплекс |
509.
| омплекс | Ќазвание комплекса | ќсобенности |
| H2[Pt(CN)6] | ¬одорода гексацианоплатинат(IV) | ислота, анионное, ацидокомплексное. |
| [Co(SO4)(NH3)5]NO3 | ѕентаамминсульфаткобальта(III) нитрат | —оль, катионное, смешанное. |
| [Pd(H2O)4]Cl2 | “етрааквапалладий(II)хлорид | —оль, катионное, аквакомплекс |
| [Cd(NH3)4](OH)2 | “етраамминкадмий(II) гидроксид | ќснование, катионное, амминокомплексное |
510. —ульфат меди CuSO4, вода H2O, газообразный аммиак NH3. Ќеобходимо растворить сульфат меди, пропустить через раствор аммиак - образуетс€ комплекс:
CuSO4(тв) + H2O = CuSO4(ж),
CuSO4(ж) + NH3(г) = [Cu(NH3)4](SO4)
511. Ќитрат ртути Hg(NO3)2, вода H2O, иодид кали€ KI. Ќеобходимо растворить соли в воде, к нитрату ртути добавить иодид кали€ - выпадет осадок. ѕрилить иодида до полного растворени€ осадка - образовалась комплексна€ соль:
Hg(NO3)2(тв) + H2O = Hg(NO3)2(ж),
KI(тв) + H2O = KI(ж),
Hg(NO3)2(ж) + KI(ж) = HgI2(тв) + 2KNO3.
HgI2(тв) + 2 KI(ж) = K2[HgI4]
512. Co3+, NH3, NO2-, K+
| омплекс | Ќазвание |
| [Co(NH3)6](NO2)3 | √ексаамминкобальт(III) нитрит |
| [Co(NH3)5(NO2)](NO2)2 | ѕентаамминнитритокобальт(III) нитрит |
| [Co(NH3)4(NO2)2](NO2) | “етраамминдинитритокобальт(III) нитрит |
| [Co(NH3)3(NO2)3] | “риамминтринитритокобальт(III) |
| K[Co(NH3)2(NO2)4] | али€ диамминтетранитритокобальтат(III) |
| K2[Co(NH3)(NO2)5] | али€ амминпентанитритокобальтат(III) |
| K3[Co(NO2)6] | али€ гексанитритокобальтат(III) |
513. ѕоскольку из раствора комплекса осаждаетс€ сульфат бари€, то барий находитс€ во внешней сфере комплекса: Ba[Cu(SCN)2(CN)2] - бари€ дицианодироданокупрат(II).
|
|
|
Ba[Cu(SCN)2(CN)2] + H2SO4 = BaSO4 + H2[Cu(SCN)2(CN)2]
514. ѕоскольку комплекс реагирует с нитратом серебра в соотношении 1:1, то во внешней сфере комплекса находитс€ 1 атом хлора: [Pt(NH3)3Cl3]Cl - триамминдихлорплатина(IV). [Pt(NH3)3Cl3]Cl + AgNO3 = AgCl + [Pt(NH3)3Cl3]NO3.
515. ƒанные сложные соединени€ представл€ют собой комплексные соли, различающиес€ между собой устойчивостью. ѕервый комплекс K[Fe(SO4)2] малоустойчив, т.е. в его растворе присутствуют катионы железа, а второй комплекс железа K3[Fe(CN)6] устойчив настолько, что в растворе нет катионов железа, способных реагировать с роданидом кали€. Fe3+ + 3CNS- = Fe(CNS)3 - красный роданид железа.
516. ѕри хранении на воздухе сульфат меди превращаетс€ в комплекс, который имеет синюю окраску (медный купорос): CuSO4 + 5H2O = [Cu(H2O)5]SO4.
517. √ексацианоферрат(II) кали€ и сульфат меди: K4[Fe(CN)6] + CuSO4 = Cu2[Fe(CN)6]. ќбразуетс€ комплексна€ соль - гексацианоферрат(II) меди, котора€ нерастворима. [Fe(CN)6]+4 + Cu+2 = Cu2[Fe(CN)6].
518. 5K4[Fe(CN)6] + KMnO4 + 4H2SO4 = 5K3[Fe(CN)6] + MnSO4 + 3K2SO4 + 4H2O. ќбразовалс€ K3[Fe(CN)6] - гексацианоферрат(III) кали€.
519. ѕрочность комплекса, согласно электростатической теории, определ€етс€ зар€дом и радиусом комплексообразовател€ Ц чем они больше, тем прочнее комплекс; а также силой лигандов Ц чем сильнее лиганд, тем прочнее комплекс. “ак, в случае а) прочнее [Co(CN)6]3- , т.к. z (Co = 3) > z (Co = 2). ¬ случае б) прочнее [Co(CN)6]4-, т.к. CN- - лиганд сильного пол€. в) прочнее [ZrF6]2- , т.к. r (Ti)< r (Zr).
520. ѕрочность комплекса, согласно электростатической теории, определ€етс€ зар€дом и радиусом комплексообразовател€ Ц чем они больше, тем прочнее комплекс; а также силой лигандов Ц чем сильнее лиганд, тем прочнее комплекс. “ак, в случае а) прочнее [Zn(NH3)4]2- , т.к. NH3 - лиганд сильного пол€. ¬ случае б) прочнее [Hg(CN)4]2-, т.к. CN- - лиганд сильного пол€. в) прочнее [Cu(NH)3]2+ , т.к. z (Cu2+)< z (Cu+).
521. ѕрочность комплекса, согласно электростатической теории, определ€етс€ зар€дом и радиусом комплексообразовател€ Ц чем они больше, тем прочнее комплекс; а также силой лигандов Ц чем сильнее лиганд, тем прочнее комплекс. “ак, в случае а) прочнее [Zn(—N)4]2- , т.к. CN - лиганд сильного пол€. ¬ случае б) прочнее [BeF4]2-, т.к. F Ц ионы более сильные лиганды, чем Cl-ионы. в) прочнее [Zr(CN)6]2- , т.к. z (Ti+4)< z (Zr4+).
522. н
523. ¬заимодействие между центральным атомом Ni и лигандами (NH3) происходит по донорно-акцепторному механизму.

|
¬ образовании гибридных облаков участвуют вакантные 4s-, 4p-, 4d-орбитали иона Ni2+ и электронные пары атомов азота. “.о., гибридизуютс€ одна s-, три р-орбитали и две 4d-орбитали, тип гибридизации комплекса: sp3d2, геометри€: октаэдр, парамагнетизм комплекса определ€етс€ наличием 2 неспаренных электронов на 3d-орбитали атома никел€.
|
|
|
524.

|
¬заимодействие между центральным атомом Ni и лигандами (Cl) происходит по донорно-акцепторному механизму. ¬ образовании гибридных облаков участвуют вакантные 4s- и 4p- орбитали иона Ni2+ и электронные пары атомов хлора. “.о., гибридизуютс€ одна s- и три р-орбитали, тип гибридизации комплекса: sp3, геометри€: тетраэдр, парамагнетизм комплекса определ€етс€ наличием 2 неспаренных электронов на 3d-орбитали атома никел€.[Ni(CN)4]2- ¬заимодействие между центральным атомом Ni и лигандами (CN) происходит по донорно-акцепторному механизму. ¬ образовании гибридных облаков участвуют вакантные 4s- и 4p- орбитали иона Ni2+ и электронные пары атомов азота. “.о., гибридизуютс€ одна s-, две р-орбитали и одна 3d-орбиталь, тип гибридизации комплекса: dsp2, геометри€: плоский комплекс. “.к. на 3d-орбитал€х атома никел€ нет неспаренных электронов, то комплекс диамагнитен.
525.  ¬заимодействие между центральным атомом Fe и лигандами (CN) происходит по донорно-акцепторному механизму. ¬ образовании гибридных облаков участвуют вакантные3d-, 4s- и 4p- орбитали иона Fe2+ и электронные пары атомов азота. “.о., гибридизуютс€ одна s-,три р- и две d- орбитали, тип гибридизации комплекса: d2sp3, геометри€: октаэдр. Ќа 3d - орбитал€х атома железа нет неспаренных электронов, поэтому комплекс диамагнитен.
¬заимодействие между центральным атомом Fe и лигандами (CN) происходит по донорно-акцепторному механизму. ¬ образовании гибридных облаков участвуют вакантные3d-, 4s- и 4p- орбитали иона Fe2+ и электронные пары атомов азота. “.о., гибридизуютс€ одна s-,три р- и две d- орбитали, тип гибридизации комплекса: d2sp3, геометри€: октаэдр. Ќа 3d - орбитал€х атома железа нет неспаренных электронов, поэтому комплекс диамагнитен.
526.
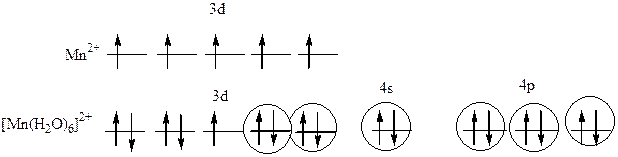
|
¬заимодействие между центральным атомом Mn и лигандами (Ќ2ќ) происходит по донорно-акцепторному механизму. ¬ образовании гибридных облаков участвуют вакантные3d-, 4s- и 4p- орбитали иона Mn2+ и электронные пары атомовкислорода. “.о., гибридизуютс€ одна s-,три р- и две d- орбитали, тип гибридизации комплекса: d2sp3, геометри€: октаэдр. Ќа 3d - орбитал€х атома марганца есть один неспаренный электрон, поэтому комплекс парамагнитен.
527.
528. ÷ветность комплексов объ€сн€етс€ наличием неспаренных электронов на расщепленных под действием лигандов d-орбитал€х. Ќеспаренный электрон может возбуждатьс€ под действием кванта света и переходить на свободную расщепленную орбиталь Ц соединение приобретает цветность. “ак,
529. —ила лиганда в комплексе определ€ет его спин. ≈сли лиганд слабого пол€, как в случае с комплексом [CoF6]3-, то заполнение расщепленных d-подуровней происходит по правилу ’унда, вкруговую. ≈сли лиганд сильного пол€, как в [Co(NH3)6]3+ , то расщепленные подуровни заполн€ютс€ против правила, сначала низ, а за тем верх, и комплекс имеет низкий спин.
рисунок
530. ¬ случае лиганда сильного пол€, например —ќ, NH3, расщепленные d-подуровни центрального атома заполн€ютс€ против правила ’унда, сначала низ, а за тем верх. Ёто случай а). Ќапример, [Fe(NH3)4]2-. ¬ случае лиганда слабого пол€, например F-, OH-, заполнение расщепленных d-подуровней происходит по правилу ’унда, вкруговую. Ёто случай б). Ќапример, [CoF6]3 .
—пособы выражени€ концентрации растворов.
531. —пособы выражени€ концентрации растворов: массова€, мольна€ и объемна€ доли, мол€рна€ концентраци€, мол€рна€ концентраци€ эквивалентов, титр, мол€льность. ћассова€ дол€ растворенного вещества - количество грамм растворенного вещества, содержащеес€ в 100 граммах раствора. Ќапример, концентраци€ спирта 40% означает, что в 100 граммах водки содержитс€ 40 грамм спирта. 1%-ный раствор иода означает, что 100 грамм раствора содержат 1 грамм иода.
| ƒано V(раствора)=10л. W%(KOH)= 40% ρ = 1.40 г.мл | Ќайдем массу раствора: m = V*ρ = 10000*1.4 = 14000 г. ¬ растворе должно содержатьс€: m(KOH) = 14000*40%/100% = 5600 грамм, m(H2O) = 14000 - 5600 = 8400 грамм. |
532.
| ƒано: m(раствора)=5 кг. W%(FeSO4)= 40% | FeSO4*7H2O = FeSO4(раствор). 5 кг раствора с массовой долей FeSO4 равной 1% содержитс€ соли: m(FeSO4) = 5000*1%/100% = 50 грамм. n(FeSO4) = m/M = 50/152 = 0.33 моль = n(FeSO4*7H2O) m(FeSO4*7H2O) = n*M = 0.33*278 = 91.74 грамм. |
533.
|
|
|
| ƒано: V(раствора)=1 л W%(HCl)= 30% ρ = 1.15 г.мл | Ќайдем массу раствора: m = V*ρ = 1000*1.15 = 1150 г. ¬ растворе должно содержатьс€: m(HCl) = 1150*30%/100% = 345 грамм, m(H2O) = 1150 - 345 = 805 грамм. |
534. —пособы выражени€ концентрации растворов: массова€, мольна€ и объемна€ доли, мол€рна€ концентраци€, мол€рна€ концентраци€ эквивалентов, титр, мол€льность. ћол€рна€ концентраци€ растворенного вещества - количество моль растворенного вещества, содержащеес€ в 1 литре раствора. ћол€рна€ концентраци€ эквивалента растворенного вещества - количество моль эквивалентов растворенного вещества, содержащеес€ в 1 литре раствора. Ќапример, концентраци€ соли 1 моль/л или 1 моль-экв/л означает, что в 1 литре раствора содержитс€ 1 моль или 1 моль эквивалентов соли.
| ƒано: V(H2SO4)=500 мл. m(H2SO4) = 196 г. | n(H2SO4) = m/M = 196/98 = 2 моль nэ(H2SO4) = m/Mэ = 196/49 = 4 моль —м(H2SO4) = n/V = 2/0.5 = 4 моль/л, —н(H2SO4) = nэ/V = 4/0.5 = 8 моль-экв/л, |
535.
| ƒано: —м(Al2(SO4)3)=0.1 моль/л V(раствора) = 2 литра |  m(Al2(SO4)3) = —м*V*ћ = 0.1*2*342 = 68.4 г.
Ё(Al2(SO4)3) = 6, следовательно, —э = 6*—м = 0.6 моль-экв/л
m(Al2(SO4)3) = —м*V*ћ = 0.1*2*342 = 68.4 г.
Ё(Al2(SO4)3) = 6, следовательно, —э = 6*—м = 0.6 моль-экв/л
|
536.
| ƒано: —м(NH3) = 18 моль/л V(раствора) = 1 литр |  n(NH3) = —м*V = 18*1 = 18 моль.
V(NH3) = n*Vм = 36*22.4 = 806.4 литра.
n(NH3) = —м*V = 18*1 = 18 моль.
V(NH3) = n*Vм = 36*22.4 = 806.4 литра.
|
537.
| ƒано: а) —э(Na2CO3) = 0.25 н V(раствора) = 0.5 литра б) m(CuSO4) = 8 г. Cэ = 0.1 н |  а) nэ(Na2CO3) = —э*V = 0.25*0.5 = 0.125 моль-экв. ћэ(Na2CO3) = 53 г/моль-экв. m(Na2CO3) = nэ*ћэ = 0.125*53 = 6.625 г.
б) V = m/(Mэ*Cэ) = 8/(80*0.1) = 1 литр.
а) nэ(Na2CO3) = —э*V = 0.25*0.5 = 0.125 моль-экв. ћэ(Na2CO3) = 53 г/моль-экв. m(Na2CO3) = nэ*ћэ = 0.125*53 = 6.625 г.
б) V = m/(Mэ*Cэ) = 8/(80*0.1) = 1 литр.
|
538. ћол€льность - количество моль растворенного вещества, приход€щеес€ на 1 кг. растворител€.
| ƒано: m(глицерина) = 460 г. V(воды) = 5 л. | n(глицерина) = m/M = 460/92 = 5 моль. ћол€льность —m(глицерина) = n/m(H2O) = 5 моль/5 кг = 1 моль/кг |
539. —пособы выражени€ концентрации растворов: массова€, мольна€ и объемна€ доли, мол€рна€ концентраци€, мол€рна€ концентраци€ эквивалентов, титр, мол€льность. “итр - количество грамм растворенного вещества в 1 миллилитре раствора.
| ƒано: а) —м(HCl) = 0.1 м б) —э(H2SO4) = 0.1 н в) w%(HNO3) = 31.5% p = 1.19 г/мл. | а) ¬озьмем 1 литр раствора. n(HCl) = 0.1 моль. m(HCl) = n*M = 0.1*36.5 = 3.65 г. “(HCl) = m/V = 3.65/1000 = 0.00365 г/мл. б) ¬озьмем 1 литр раствора. ѕоскольку эквивалент серной кислоты равен 2, то —м(H2SO4) = 1/2—э(H2SO4) = 0.05 моль/л. n(H2SO4) = 0.05*1 = 0.05 моль. m(H2SO4) = n*M = 0.05*98 = 4.9 г. “(H2SO4) = m/V = 4.9/1000 = 0.0049 г/мл в) ¬озьмем 1 литр раствора. ћасса раствора равна V*p = 1000*1.19 = 1190 г. m(HNO3) = 1190*31.5%/100% = 374.85 г. “(HNO3) = 374.85/1000 = 0.375 г/мл. |
541.
| ƒано: —м(HNO3) = 4.65 м p = 1.15 г/мл. | ¬озьмем 1 литр раствора. m(раствора) = 1000*1.15 = 1150 г. m(HNO3) = —м*ћ(HNO3)*V = 4.65*63*1 = 292.95 г. w%(HNO3) = 292.95*100%/1150 = 25.5% |
542.
| ƒано: W%(KOH) = 26 % p = 1.24 г/мл. | ¬озьмем 1 литр раствора. m(раствора) = 1000*1.24 = 1240 г.
m(KOH) = 1240*26%/100% = 322.4 г.
m(H2O) = 1240 - 322.4 = 917.6 г.
 = 322.4/(56*1) = 5.75 моль/л
ћол€льность Cm = n( ќH)/m(H2O) = 5.75/0.9176 = 6.27 моль/кг.
“итр “( ќЌ) = —м*ћ/1000 = 5.75*56/1000 = 0.322 г/мл = 322.4/(56*1) = 5.75 моль/л
ћол€льность Cm = n( ќH)/m(H2O) = 5.75/0.9176 = 6.27 моль/кг.
“итр “( ќЌ) = —м*ћ/1000 = 5.75*56/1000 = 0.322 г/мл
|
543.
| ƒано: w%(HNO3) = 36 % p = 1.22 г/мл. | а) ¬озьмем 1 литр раствора. m(раствора) = 1000*1.22 = 1220 г.
m(HNO3) = 1220*36%/100% = 439.2 г.
m(H2O) = 1220-439.2 = 780.8 г.
б)  = 439.2/(63*1) = 7 моль/л
в) “.к. Ё(HNO3) = 1, то —э = —м = 7 моль-экв/л.
г) ћол€льность Cm = n(HNO3)/m(H2O) = 7/0.7808 = 8.9 моль/кг.
д) “ = —э*ћэ/1000 = 7*63/1000 = 0.441 г/мл
е) n(H2O) = m/M = 780.8/18 = 43.4 моль.
ћольна€ дол€ N = = 439.2/(63*1) = 7 моль/л
в) “.к. Ё(HNO3) = 1, то —э = —м = 7 моль-экв/л.
г) ћол€льность Cm = n(HNO3)/m(H2O) = 7/0.7808 = 8.9 моль/кг.
д) “ = —э*ћэ/1000 = 7*63/1000 = 0.441 г/мл
е) n(H2O) = m/M = 780.8/18 = 43.4 моль.
ћольна€ дол€ N = 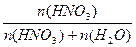 = 7/(7+43.4) = 0.14 = 7/(7+43.4) = 0.14
|
544.
|
|
|
| ƒано: w%(H2SO4) = 11.6 % p = 1.08 г/мл. ћэ(H2SO4) = 49 г-экв/моль | ¬озьмем 1 литр раствора.
m(раствора) = 1000*1.08 = 1080 г.
m(H2SO4) = 1220*11.6%/100% = 141.52 г.
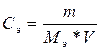 = 141.52/(49*1) = 2.9 моль-экв/л = 141.52/(49*1) = 2.9 моль-экв/л
|
545.
| ƒано: w%(NaOH) = 10 % m(раствора) = 1 кг. m(H2O) = 200 г. | m(NaOH) = m(раствора)*w%(NaOH)/100% = 1000*10/100 = 100г. ¬ растворе было 1000-100 = 900 грамм воды, а осталось 900-200 = 700 г. m(раствора) = 700+100 = 800 грамм. w%(NaOH) = 100*100%/800 = 12.5% |
546.
| ƒано: w%(соли) = 20 % m(раствора) = 400 г. m(вещества) = 50 г. | m(соли) = m(раствора)*w%(соли)/100% =400*20/100 = 80 г. ¬ растворе было 400-80 = 320 грамм воды. ќсталось соли 80-50 = 30 г. m(раствора) = 320+30 = 350 гр. w%(NaOH) = 30*100%/350 = 8.57% |
547.
| ƒано: w%1(соли) = 30 % V(H2O) = 300 мл. w%2(соли) = 10 % | ѕусть масса соли, которую необходимо добавить к воде равна х. Ќайдем массу воды, приход€щуюс€ на х грамм соли:  , откуда mТ(H2O) = 2.33х. ѕодставим х в конечную формулу дл€ 10%-ного раствора: , откуда mТ(H2O) = 2.33х. ѕодставим х в конечную формулу дл€ 10%-ного раствора:  => х=45 г. => х=45 г.
|
548.
| ƒано: V1(NaCl) = 500 мл w%1(NaCl) = 20 % p1 = 1.152 г/мл w%2(соли) = 4.5 % p2 = 1.029 г/мл | ј) Ќайдем массу сухой соли в исходном растворе: m1(раствора) = V1*p1 = 500*1.152 = 576 г. m(соли) = 576*20%/100% = 115.2 г. Ѕ) Ёта же масса соли содержитс€ в конечном растворе: m2(раствора) = m(соли)*100%/ w%2(соли) = 115.2*100/4.5 = 2560 г. V2(раствора) = m/p = 2560/1.029 = 2488 мл. |
549.
| ƒано: w%1( OH) = 20% w%2( OH) = 50% m(р-ра2) = 1 кг. w%3( OЌ) = 25% m(р-ра1) =? | ¬ыразим массу чистого гидроксида кали€, содержащегос€ в каждом из растворов: m1( ќЌ) = m(р-ра1)*w%1( ќЌ)/100% = 0.2*m(р-ра1) m2( ќЌ) = m(р-ра2)*w%2( ќЌ)/100% = 0.5 кг. m3( ќЌ) = m(р-ра3)*w%3( ќЌ)/100% = 0.25*m(р-ра3) ѕо смыслу задачи: m1( ќЌ) + m2( ќЌ) = m3( ќЌ) m(р-ра1)*0.2 + 0.5 = m(р-ра3)*0.25 m(р-ра1) + 1кг = m(р-ра3) m(р-ра1)*0.2 + 0.5 = (m(р-ра1)+1)*0.25 m(р-ра1) = 5 кг. |
550.
| ƒано: w%1(—a(NO3)2) = 10% w%2(—a(NO3)2) = 2% m(р-ра1) = 300 г. m(р-ра2) = 500 г. w%общ(—a(NO3)2) =? | Ќайдем массу чистого —a(NO3)2, в каждом из растворов:
m1(—a(NO3)2) = m(р-ра1)*w%1/100% = 300*10%/100% = 30 г.
m2(—a(NO3)2) = m(р-ра2)*w%2/100% = 500*25/100% = 10 г.
w%общ(—a(NO3)2) = 
|
551.
| ƒано: w%1(H2SO4) = 11.6% p1 = 1.08 г/мл —м2 = 0.1 моль/л V2 = 250 мл. V1 =?, V(Ќ2ќ) =? | Ќайдем массу чистой серной кислоты, содержащейс€ в 0.1м растворе: m(H2SO4) = —м*V*ћ = 0.1*0.25*98 = 2.45 г. Ёта же масса кислоты содержитс€ в 11.6%-ном растворе: m(раствора) = 2.45*100%/11.6% = 21.12 г. V(р-ра) = m/p = 21.12/1.08 = 20 мл V(Ќ2ќ) = 250-20 = 230 мл. |
552.
| ƒано: w%1(HCl) = 20% p1 = 1.10 г/мл —н2(HCl) = 0.1 моль/л V2 = 1 л. V1 =?, V(Ќ2ќ) =? | Ќайдем массу чистой HCl, содержащейс€ в 0.1н растворе: m(HCl) = —н*V*ћэ = 0.1*1*36.5 = 3.65 г. Ёта же масса кислоты содержитс€ в 20%-ном растворе: m(раствора) = 3.65*100%/20% = 18.25 г. V(р-ра) = m/p = 18.25/1.1 = 17 мл V(Ќ2ќ) = 1000-17 = 983 мл. |
553.
| ƒано: V(H2SO4) = 50 мл. —н(H2SO4) =? н V(щелочи) = 25 мл. —н(щелочи) = 0.4 н | «акон эквивалентов: —н1*V1 = —н2*V2
Cн(H2SO4) = 
|
554.
| ƒано: C(HCl) = 0.5н V(AgNO3) = 0.5л. —н(AgNO3) = 0.2 н V(HCl) =? | «акон эквивалентов: —н1*V1 = —н2*V2
V(HCl) = 
|
555.
| ƒано: V(щелочи) = 20 мл. “(щелочи) = 0.012 г/мл V(кислоты) = 24 мл. —н(кислоты) = 0.25 н ћ(щелочи) =? | «акон эквивалентов: —н1*V1 = —н2*V2
Cн(щелочи) = 
 , следовательно, ћэ(щелочи)=1000*0.012/0.3= 40 г/моль Ц это NaOH. , следовательно, ћэ(щелочи)=1000*0.012/0.3= 40 г/моль Ц это NaOH.
|
556.
| ƒано: w%(H2SO4)=20% p = 1.10 г/мл m(Mg) = 24.3 г. V(H2SO4) =? | H2SO4 + Mg = MgSO4 + H2 n(Mg) = m/M = 24.3/24.3 = 1 моль. —огласно уравнению реакции, n(Mg) = n(H2SO4) = 1 моль. m(H2SO4) = n*M = 1*898 = 98 г. m(раствора H2SO4) = 98*100%/15% = 653.3 г. V(H2SO4) = m/p = 653.3/1.1 = 594 мл. |
557.
| ƒано: w%(K2—O3)=20% V(K2—O3)=650 мл p = 1.189 г/мл —(H2SO4) =2ћ V(H2SO4) =? V(—O2) =? | H2SO4 + K2—O3= 2SO4 + —ќ2 + H2ќ
Ќайдем число моль K2—O3:
m(раствора) = V*r = 650*1/189 = 772.85 г.
m(K2—O3) = 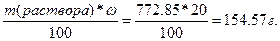 .
n(K2—O3) = m/M = 154.57/138 = 1.12 моль.
ѕо уравнению рекции: n(K2—O3) = n(H2SO4) = n(—ќ2)
V(H2SO4) = n/Cm = 1.12/2 = 560 мл.
V(—ќ2) = n*Vm = 1.12*22.4 = 25 л. .
n(K2—O3) = m/M = 154.57/138 = 1.12 моль.
ѕо уравнению рекции: n(K2—O3) = n(H2SO4) = n(—ќ2)
V(H2SO4) = n/Cm = 1.12/2 = 560 мл.
V(—ќ2) = n*Vm = 1.12*22.4 = 25 л.
|
558.
|
|
|
| ƒано: w%(NH3)=23% p =0.916 г/мл —(H—l)=6ћ V(HCl) = 750мл V(NH3) =? m(NH4Cl)=? | NH3 + HCl = NH4Cl Цобразуетс€ хлорид аммони€
n(HCl) = C*V = 6*0.75 = 4.5 моль. ѕо уравнению реакции: n(HCl) = n(NH3) = n(NH4Cl).
m(NH4Cl) = n*M = 4.5*53.5 = 240.75 г.
m(NH3) = n*M = 4.5*17 = 76.5 г.
m(раствора NH3) =  V(NH3) = m(раствора)/ r = 332.6/0.916 = 363.1 мл.
V(NH3) = m(раствора)/ r = 332.6/0.916 = 363.1 мл.
|
559.
| ƒано: V(BaCl2) = 100 мл w% = 15% V(H2SO4)= 14.4 мл Cм, —н, “ =? | H2SO4 + BaCl2 = BaSO4 + 2HCl
ѕусть плотность раствора BaCl2 = 1. Ќайдем n(BaCl2)
m(раствора BaCl2) = V*p = 100 г. m(BaCl2) = 100*15%/100% = 15 г.
n(BaCl2) = m/M = 15/208 = 0.0721 моль. = n(H2SO4)
Cм(H2SO4) = n/V = 0.0721/0.0144 = 5 моль/л
—н(H2SO4) = 2—м = 10 моль/л. ћэ(H2SO4) = 49 г/моль.
“ =  г/мл г/мл
|
560.
| ƒано: V(BaCl2) = 100 мл w% = 20% p =1.203 г/мл m (BaSO4)=? | 3BaCl2 + Cr2(SO4)3 = 3BaSO4 + 2CrCl3 Ќайдем число моль BaCl2: m(раствора BaCl2) = V*p = 100 * 1.203 = 120.3 г. m(BaCl2) = 120.3*20%/100% = 24.06 г. n(BaCl2) = m/M = 24.06 / 208 = 0.116 моль. n(BaSO4) = n(BaCl2) = 0.116 моль. m(BaSO4) = n*M = 0.116*233 = 26.9 г. |






