561.
| ƒано: m(NH4Cl) = 10 г. V(Ќ2ќ) = 233 мл. ∆T = -2.8oC —уд = 4.2 ƒж/(г* ) | n(NH4Cl) = m/M = 10*53.5 = 0.19 моль.
Qраств(NH4OH) = m(раствора)*∆“*Cуд = (10+233)*2.8*4.2 = -2.86 кƒж
∆Hoраств = 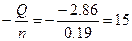 кƒж/моль. кƒж/моль.
|
562.
| ƒано: m(NaOH) = 10 г. V(Ќ2ќ) = 250 мл. ∆Hoраств(NaOH) =?. ∆“ = 6.7о— —уд = 4.2 ƒж/(г* ) |
n(NaOH) = m/M = 10*40 = 0.25 моль.
Qраств(NaOH) = m(раствора)*∆“*Cуд = (10+250)*6.7*4.2 = 7.32 кƒж
∆Hoраств = 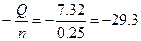 кƒж/моль. кƒж/моль.
|
563.
| ƒано: m(CaCl2) = 10 г. Q = 6.82 кƒж m(CaCl2*6H2O) = 10г. Q = -0.87 кƒж ∆Hoгидр(—aCl2) =? | n(CaCl2) = m/M = 10/111 = 0.09 моль. ∆Hoраств(CaCl2) = -Q/n = -6.82/0.09 = -75.7 кƒж/моль n(CaCl2*6Ќ2ќ) = m/M = 10/219 = 0.046 моль. ∆Hoраств(CaCl2*6Ќ2ќ) = -Q/n = 0.87/0.046 = 19.05 кƒж/моль ∆Hoгидр(—aCl2) = ∆Hoраств(CaCl2) - ∆Hoраств(CaCl2*6Ќ2ќ) = -94.75 кƒж/моль |
564.
| ƒано: ∆Hoраств(CuSO4*5H2O)=11.72 кƒж/моль. V(Ќ2ќ) = 0.2 литра. ∆“ = 2о— —уд = 4.2 ƒж/(г* ) m(CuSO4) = 4 г. | n(CuSO4) = m/M = 4/160 = 0.025 моль Q = m(раствора)*∆“*Cуд = (200+4)*2*4.2 = 1.71 кƒж ∆Hoраств(CuSO4) = -Q/n = Ц 1.71/0.025 = -68.5 кƒж/моль ∆Hoгидр(—uSO4) = ∆Hoраств(CuSO4) - ∆Hoраств(CuSO4*5Ќ2ќ) = -68.5 Ц 11.72 = -80.22 кƒж/моль |
565.
| ƒано: ∆Hoрастворени€(NH4NO3)=26.32 кƒж/моль. V(Ќ2ќ) = 0.2 литра. ∆“ = 5о— —уд = 3.77 ƒж/(г* ) m(NH4NO3) =? | Qраств(NH4NO3) = m(H2O)*∆“*Cуд = 200*5*3.77 = 3.77 кƒж
n = 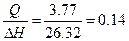 моль.
m(NH4NO3) = n*M = 0.14*80 = 11.46 грамм. моль.
m(NH4NO3) = n*M = 0.14*80 = 11.46 грамм.
|
566.
| ƒано: m(H2O) = 25 г m(NaCl) = 8,75 г S -? | –ешение: 8,75 г NaCl Ц 25 г Ќ2ќ х г NaCl - 100 г Ќ2ќ х = 8,75*100/25 = 35 г NaCl/100г Ќ2ќ |
567.
| ƒано: V(Ќ2ќ) = 50 мл. n(Zn(NO3)2) = 0.003 моль | m(Zn(NO3)2) = n*M = 0.003*189 = 0.567 г.
–астворимость S = 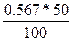 = 1.13 г/100 г воды = 1.13 г/100 г воды
|
568.
| ƒано: w(K2Cr2O7) = 45.2%. S -? | ¬озьмем 100 г раствора: m(K2Cr2O7) = 45.2 г, m(H≠2O) = 100 Ц45.2 = 54.8 г.
–астворимость S = 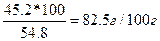
|
569.
| ƒано: S (KNO3) = 31.6 г/100 г w -? | ¬озьмем раствор, в котором m(KNO3) = 31.6 г, тогда m(H≠2O)=100 г.
w (KNO3) = 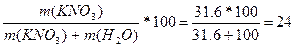 % %
|
570.
| ƒано: S (KNO3) = 55 г/100 г m(р-ра) = 60 г. m (KNO3) -? |
w (KNO3) = 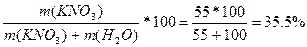 %
¬ 60 г раствора: m(KNO3) = (60*35.5) / 100 = 21.3 г. %
¬ 60 г раствора: m(KNO3) = (60*35.5) / 100 = 21.3 г.
|
571.
| ƒано: S1(K2CO3)= 155 г/100г S2(K2CO3)= 111 г/100г m(раствора) = 770 г. | Ќайдем, сколько грамм соли содержитс€ в 770 граммах раствора при 100о—. m1(K2CO3) = 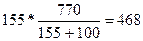 грамм, следовательно воды в растворе содержитс€ 770 Ц 468 = 302 грамма. Ќайдем, сколько соли может растворитс€ в 302 г. воды при 00—: m2(K2CO3) = грамм, следовательно воды в растворе содержитс€ 770 Ц 468 = 302 грамма. Ќайдем, сколько соли может растворитс€ в 302 г. воды при 00—: m2(K2CO3) = 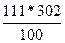 =335г.
“.е. при охлаждении из раствора выделитс€ 468 Ц 335 = 133 гр. соли. =335г.
“.е. при охлаждении из раствора выделитс€ 468 Ц 335 = 133 гр. соли.
|
572.
|
|
|
| ƒано: S1(Pb(NO3)2)= 90 г/100г S2(Pb(NO3)2)= 46 г/100г V(H2O) = 200 мл T = 200C | ѕри 60о— в 200г воды раствор€етс€ m1(Pb(NO3)2) = 90*200/100 = 180г ѕри 0о— в 200г воды раствор€етс€ m1(Pb(NO3)2) = 46*200/100 = 92 г. ¬ осадок выпадет m1-m2 = 180 Ц 192 = 92 грамма соли. |
573.
| ƒано: “1 = 100о—, “2 = 20о— S1(NaNO3) = 176 г/100г S2(NaNO3) = 88 г/100г m(NaNO3) = 120 г. | Ќайдем массовую долю соли в растворе при обеих температурах: w1% = 176*100%/(176+100) = 63,8%; w2% = 88*100%/(88+100) = 46,8% ѕусть m1(р-ра) = х, m2(р-ра) = у, m1(NaNO3) = z, m2(NaNO3) = k. ѕо условию: х-у = 120, z-k = 120 грамм. x = z*100%/w1% = z*1.57, y = k*100%/w2% = k*2.14. ѕолучаем систему уравнений: z*1.57 Ц k*2.14 = 120иz-k =120. –еша€ ее, получаем х = 357,4 грамм; z = 228 грамм. |
574.
| ƒано: S(H2S) = 2.91 P = 101325 ѕа T = 200C W%(CO2) =? | ¬ одном литре воды раствор€етс€ 2.91 литра H2S.
m(H2O) = 1000 г.
n(H2S) = V/Vм = 2.91/22.4 = 0.13 моль
m(H2S) = n*M = 0.13*34 = 4.42 г.
w%(H2S) = 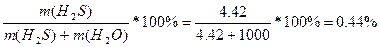
|
575.
| ƒано: S(CO2) = 0.878 P =? W%(CO2) = 1 % | ¬ одном литре воды раствор€етс€ 0.878 литр диоксида углерода. m(H2O) = 1000 г. n(CO2) = V/Vм = 2.91/22.4 = 0.13 моль m(CO2) = n*M = 0.13*44 = 5.72 г. |
576.
| ƒано: W%(C12H22O11) = 10% “ = 100о— –о = 101.3*105 ѕа –Т =? | –о - –Т = –о 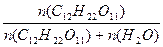 ¬озьмем 1 кг раствора. m(C12H22O11) = 100 г.
n(C12H22O11) = m/M = 100/342 = 0.292 моль
m(H2O) = 1000-100 = 900 г.
n(H2O) = 900/18 = 50 моль.
101.3*105 - –Т = 101.3*105
¬озьмем 1 кг раствора. m(C12H22O11) = 100 г.
n(C12H22O11) = m/M = 100/342 = 0.292 моль
m(H2O) = 1000-100 = 900 г.
n(H2O) = 900/18 = 50 моль.
101.3*105 - –Т = 101.3*105 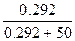 –Т=1.007*105 ѕа = 755.6 мм.рт.ст. –Т=1.007*105 ѕа = 755.6 мм.рт.ст.
|
577.
| ƒано: –0 = 8199.325 ѕа V(H2O) = 540 мл m(C6H12O6) = 1 г. PТ =? | Po - PТ = P0*N(C6H12O6)
n(H2O) = m/M = 540/18 = 30 моль.
n(—6H12O6) = m/M = 36/180 = 0.2 моль.
8199.325 - PТ = 8199.325* 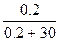 , следовательно, PТ = 8145.025 ѕа , следовательно, PТ = 8145.025 ѕа
|
578.
| ƒано: –0 = 23939.35 ѕа PТ = 21854.4 ѕа m(ацетона) = 200 г. m(неэлект.)=10.5 г. ћ =? | Po - PТ = P0*N2
n(ацетона) = m/M = 200/58 = 3.45 моль.
.
23939.35 Ц 21854.4 = 23939.35* 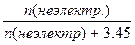 , следовательно, n (неэлектр.) = 0.329 моль, ћ(неэлектр.) = m / n = 10.5 / 0.329 = 32 г/моль. , следовательно, n (неэлектр.) = 0.329 моль, ћ(неэлектр.) = m / n = 10.5 / 0.329 = 32 г/моль.
|
579.
| ƒано: –0 = 47375 ѕа PТ = 33310 ѕа n(в-ва) = 1 n(H2O) =? | Po - PТ = P0*N(вещества) => 47375 - 33310 = 47375* 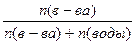 1+n(H2O) = 3.37 => n(H2O) = 2.37 моль.
1+n(H2O) = 3.37 => n(H2O) = 2.37 моль.
|
580.
| ƒано: w(—2Ќ5ќЌ)=25% “зам-? | Ќ2ќ*—м, где —м-мол€льность р-ра или моль в-ва на 1кг растворител€. Ќ2ќ = 1,86. ¬озьмем 100 грамм раствора, тогда масса этанола будет 0,25*100 = 25 г, а количество в-ва его n = m/M = 25/46 = 0,54 моль. ћасса воды тогда будет 100-25 = 75 грамм. “огда —м = n(C2H5OH)/M(H2O) = 0,543/75*10-3 = 7,25 моль/кг. D“зам = 1,86*7,25 = 13,480. “зам = -13,480— |
581.
| ƒано: W%(C6H12O6) = 10% “(кип) =? | Δ“(кип) = ≈(Ќ2ќ)*—m, где —m - мол€льна€ концентраци€. Ќайдем мол€льность раствора глюкозы: возьмем 100 грамм раствора m(C6H12O6) = 10 г. n(C6H12O6) = m/M = 10/180 = 0.056 моль m(H2O) = 100-10 = 90 г. —m = n(C6H12O6)/m(H2O) = 0.056/0.090 = 0.62 моль/кг. Δ“(кип) = ≈(Ќ2ќ)*—m = 0.52*0.62 = 0.320. “(кип) = 100.32 0— |
582.
| ƒано: m(x) = 1.6 г. V(H2O) = 250 мл. “(зам) = -0.2 о— ћ(х) =? | Δ“(зам) = (Ќ2ќ)*—m = 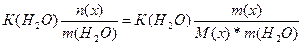 0.2 = 1.86*
0.2 = 1.86* 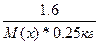 , следовательно, ћ(х) = 59.52 г/моль , следовательно, ћ(х) = 59.52 г/моль
|
583.
|
|
|
| ƒано: m(x) = 27 г. V(H2O) = 1000 мл. “(кип) = 100.78 о— ћ(х) =? | Δ“(кип) = ≈(Ќ2ќ)*—m = 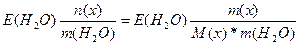 0.78 = 0.52*
0.78 = 0.52* 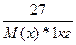 , следовательно, ћ(х) = 18 г/моль , следовательно, ћ(х) = 18 г/моль
|
584.
| ƒано: m(Ix) = 9.2 г. m(CH3OH) = 100 г. “о(кип) = 64.7 о— “Т(кип) = 65 о— ћ(Iх) =? ≈(CЌ3ќH) = 0.84 | Ќеобходимо найти мол€рную массу иода, растворенного в метаноле
Δ“(кип) = ≈(CЌ3ќH)*—m = 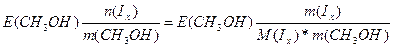 65 - 64.7 = 0.84*
65 - 64.7 = 0.84* 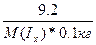 , следовательно, ћ(Iх) = 257.6 г/моль
ћолекула иода состоит из 257.6/127 = 2 атомов. , следовательно, ћ(Iх) = 257.6 г/моль
ћолекула иода состоит из 257.6/127 = 2 атомов.
|
585.
| ƒано: m(Sx) = 0.81 г. m(C6H6) = 100 г. Δ“(кип) = 0.081 о— ћ(Sх) =? ≈(C6H6) = 2.57 | Ќеобходимо найти мол€рную массу серы, растворенной в бензоле
Δ“(кип) = ≈(C6H6)*—m = 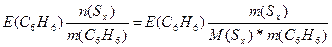 0.081 = 2.57*
0.081 = 2.57* 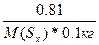 , следовательно, ћ(Sх) = 257 г/моль
ћолекула серы состоит из 257/32 = 8 атомов. , следовательно, ћ(Sх) = 257 г/моль
ћолекула серы состоит из 257/32 = 8 атомов.
|
586.
| ƒано: V(H2O) = 30 л. V(C3H5(OH)3) = 9 л. “(зам) =? p(глицерина) = 1,261 г/мл | Δ“(зам)= (Ќ2ќ)*—m= 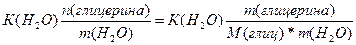 ћасса глицерина равна m = V*p = 9000*1.261 = 11349 г.
Δ“(зам) = 1.86*
ћасса глицерина равна m = V*p = 9000*1.261 = 11349 г.
Δ“(зам) = 1.86* 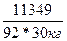 = 7.65о. –аствор замерзает при -7.65о— = 7.65о. –аствор замерзает при -7.65о—
|
587.
| ƒано: “(зам) = -150— m(C2H4(OH)2)=? | Δ“(зам)= (Ќ2ќ)*—m= 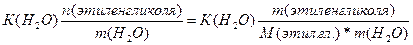 —m =
—m = 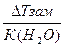 = = 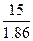 =8.06 моль / кг растворител€.
m(этил.гл.) = —m * ћ(этил.гл.) * m(H2O) = 8.06*62*1 = 499.7 г = 0.5 кг. =8.06 моль / кг растворител€.
m(этил.гл.) = —m * ћ(этил.гл.) * m(H2O) = 8.06*62*1 = 499.7 г = 0.5 кг.
|
588.
| ƒано: Pосм =? w%(сахара) = 4 % “ = 20о— = 293 p = 1.014 г/мл | Pосм = —м*R*T ¬озьмем 1 литр раствора и найдем —м сахара: m(раствора) = V*p = 1000*1.014 = 1014 г. m(сахара) = 1014*4%/100% = 40.56 г. —м(сахара) = m/(M*V) = 40.56/(342*1) = 0.12 моль/л –осм = 0.12*8.31*293 = 288.8 кѕа, –осм = 0.12*0.082*293 = 2.85 атм. |
589.
| ƒано: V(H2O) = 1 л. Pосм = 607950 ѕа m(глюкозы) = 45 г. “ =? | Pосм = —м*R*T = 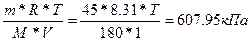 , следовательно “=293.6 , следовательно “=293.6
|
590.
| ƒано: W%(C) = 39.56 % W%(H) = 7.69 % W%(O) = 52.75 % V(H2O) = 1 л. Pосм = 9.00*105 ѕа m(маннита) = 72 г. “ = 00— = 273 | Pосм = —м*R*T = 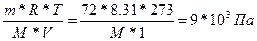 ћ(маннита) = 181 г/моль
¬озьмем 1 моль маннита. ћасса одного моль маннита 181 грамм. ¬ них содержитс€:
m(C) = 39.56%*181/100% = 72 г. n(—) = m/M = 72/12 = 6 моль
m(Ќ) = 7.69%*181/100% = 14 г. n(Ќ) = m/M = 14/1 = 14 моль
m(ќ) = 52.75%*181/100% = 96 г. n(ќ) = m/M = 96/16 = 6 моль
‘ормула маннита: —6Ќ14ќ6
ћ(маннита) = 181 г/моль
¬озьмем 1 моль маннита. ћасса одного моль маннита 181 грамм. ¬ них содержитс€:
m(C) = 39.56%*181/100% = 72 г. n(—) = m/M = 72/12 = 6 моль
m(Ќ) = 7.69%*181/100% = 14 г. n(Ќ) = m/M = 14/1 = 14 моль
m(ќ) = 52.75%*181/100% = 96 г. n(ќ) = m/M = 96/16 = 6 моль
‘ормула маннита: —6Ќ14ќ6
|
–астворы электролитов
591. Ёлектролитами называютс€ вещества, диссоциирующие в растворе. —огласно теории электролитической диссоциации, кислоты- вещества, которые при диссоциации дают катионы водорода. ќсновани€ при диссоциации дают ќЌ-группы. —оли диссоциируют на катион и анион кислотного остатка. ислые и основные соли называют промежуточными соединени€ми, т.к. они €вл€ютс€ продуктами неполной реакции нейтрализации.
592. ¬озможность электролитической диссоциации определ€етс€ природой вещества (сильный или слабый электролит), природой растворител€, температурой и т.п. »онизирующую силу растворител€ характеризует диэлектрическа€ проницаемость: вода Ц 81.0, спирт Ц 25.8, бензол Ц 2.23, фтороводород Ц 32.3. ƒиссоциаци€ NaCl:
NaCl + H2O = 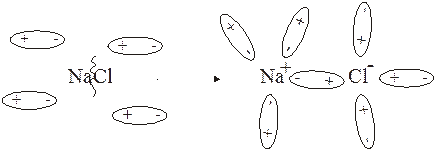
|
593. ¬ воде хлорид натри€ диссоциирует на ионы практически полностью, а спирт практически не диссоциирует. “.к. ионы, образующиес€ при диссоциации, провод€т электрический ток, то раствор NaCl проводит электрический ток, а раствор CH3CH2OH не проводит.
594. ƒл€ количественной характеристики электролитической диссоциации примен€ютс€ константа диссоциации ( д) и степень диссоциации (а). д Ц отношение произведени€ концентраций продуктов к произведению концентраций исходных веществ. а Ц отношение количества диссоциированных молекул к исходному количеству молекул. ѕосто€нной величиной при различных концентраци€х €вл€етс€ д.
595. ѕо величине электролитической диссоциации электролиты дел€тс€ на: сильные ( д >>1), средние (1> д > 10-2) и слабые ( д << 1). первым относитс€ HNO3( д = 43.6): HNO3 = H+ + NO3- . о вторым Ц HIO4 ( д = 2/3*10-2): HIO4 = H+ + IO4-. слабым электролитам относ€т NH4OH ( д = 1.76*10-5): NH4OH = NH4+ + OH-.
|
|
|
596.
| K2SO4↔ K+ + KSO4-1 KSO4-1 ↔ K+ + SO4-2 | K1 = 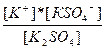 K2=
K2= 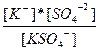
|
| Ca(OH)2 ↔ Ca2+ + 2OH - | K = 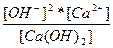
|
| H3PO4 ↔ 3H+ + PO43- | K = 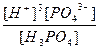
|
| KHCO3 ↔ K+ + H+ + CO32- | K = 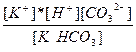
|
| 3H+ + AlO3-3↔Al(OH)3 ↔ Al3+ + 3OH- | K = 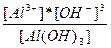
|
| Fe(OH)Cl2 ↔ Fe2+ + OH - + 2Cl - | K = 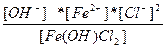
|
597.
| Ba(OH)2 ↔ Ba+2 + 2OH- | K = 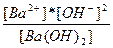
|
| H3AsO4 ↔ 3H+ + AsO4-3 | K = 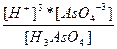
|
| KHSO4 ↔ K+ + HSO4 Ц HSO4 Ц ↔ H+ + SO4 Ц2 | K 1= 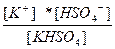 K 2=
K 2= 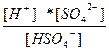
|
| Fe(OH)2Cl ↔ Fe3+ + 2OH- + Cl- | K = 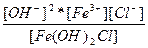
|
| 2H+ + ZnO22-↔Zn(OH)2 ↔ Zn2+ + 2OH- | K = 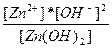
|
598.
| —a(NO3)2 ↔ Ca+2 + 2NO3- | K = 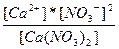
|
| Sr(OH)2 ↔ Sr+2 + 2OH- | K = 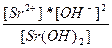
|
| NaH2PO4 ↔ Na+ + H2PO4 Ц H2PO4 Ц ↔ 2H+ + PO4 Ц3 | K 1= 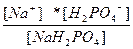 K 2=
K 2= 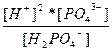
|
| 2H+ + BeO22-↔Be(OH)2 ↔ Be+2 + 2OH- | K = 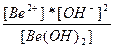
|
| AlOH(NO3)2 ↔ Al3+ + OH- + 2NO3- | K = 
|
599.
| K2Cr2O7 ↔ 2K+ + Cr2O72- | K = 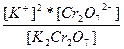
|
| H2SeO3 ↔ 2H+ + SeO32- | K = 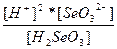
|
| NaHS ↔ Na+ + HS- HS- = H+ + S2- | K1 = 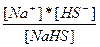 K2 =
K2 = 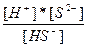
|
| Al(OH)2NO3 ↔ Al3+ + 2OH- + NO3- | K = 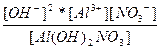
|
| 2H+ + SnO22-↔Sn(OH)2 ↔ Sn2+ + 2OH- | K = 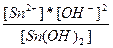
|
600.
| LiOH ↔ Li+ + OH- | K = 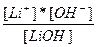
|
| H2S ↔ H+ + HS- HS- ↔ H+ + S2- | K1 = 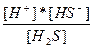 K2 =
K2 = 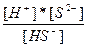
|
| K2HPO4 ↔ 2K+ + H+ + PO43- | K = 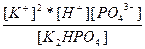
|
| Cr(OH)2Cl ↔ Cr3+ + 2OH- + Cl- | K = 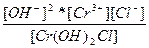
|
| 4H+ + SnO44-↔Sn(OH)4 ↔ Sn4+ + 4OH- | K = 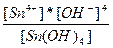
|
601. “ип диссоциации гидроксида зависит от соотношени€ энергии св€зи металл-кислород и кислород-водород. ¬ NaOH радиус атома натри€ относительно большой, поэтому энерги€ св€зи Na-O ниже O-H и он диссоциирует по типу основани€ Na-O-H = Na+ + OH-; ¬ HNO3 радиус атома азота относительно небольшой, поэтому энерги€ св€зи N-O выше O-H и он диссоциирует по типу кислоты H-O-NO≠2 = H+ + NO3- ; ¬ Zn(OH)2 радиус атома цинка средний между азотом и натрием, поэтому энергии св€зи Zn-O и O-H примерно равны, в результате соединение диссоциирует и по типу кислоты и по типу основани€ Zn2+ + 2OH- = Zn(OH)2 = 2H+ + ZnO22-. ¬ сильно кислой среде азотна€ кислота способна про€вл€ть основные свойства: H-O-NO≠2 = ќH- + NO2+.
602. —ила кислоты определ€етс€ константой ее диссоциации. „ем сильнее диссоциирует кислота, тем сильнее кислота. „ем слабее св€зь атома водорода с атомом кислорода в молекуле кислоты, тем сильнее кислота. „ем выше степень окислени€ центрального атома в однотипных кислотах, тем сильнее кислота. „ем больше атомов кислорода, св€занных только с центральным атомом приходитс€ на атомы водорода, тем сильнее кислота.
| ј) HNO2 Ц HNO3 | —ила HNO3 выше, т.к. степень окислени€ азота в ней +5, а в HNO2 cтепень окислени€ азота +3 |
| Ѕ) H2SO4 Ц H2SeO4 | –адиус атома Se больше радиуса атома S, поэтому св€зь Ќ-ќ в серной кислоте слабее св€зи Ќ-ќ в селеновой кислоте, т.е. серна€ кислота сильнее селеновой. |
| ¬) HPO3 Ц H3PO4 | “.к. в HPO3 количество атомов кислорода, св€занных только с центральным атомом, приход€щихс€ на атомы водорода, больше, чем в H3PO4, то сильнее HPO3. |
603. —ила кислоты определ€етс€ константой ее диссоциации. „ем сильнее диссоциирует кислота, тем сильнее кислота.
| ј) HF Ц HCl Ц HBr Ц HI | ¬ данном р€ду сила кислот увеличиваетс€ от HF к HI, т.к. с ростом радиуса атома галогена св€зь галогенЦводород ослабевает и кислота легче диссоциирует: HCl = H+ + Cl- |
| Ѕ) HClO4 Ц HClO3 Ц HClO2 Ц HClO | —огласно правилу, чем больше атомов кислорода, св€занных только с центральным атомом приходитс€ на атомы водорода, тем сильнее кислота. ѕоэтому в данном р€ду сила кислот уменьшаетс€. HClO = H+ + ClO- |
| ¬) HClO Ц HBrO Ц HIO | — ростом радиуса атомов галогенов от Cl к I ослабевает св€зь галоген-кислород, т.е. увеличиваетс€ константа диссоциации по основному типу. —ледовательно, сила кислот уменьшаетс€. HBrO = H+ + BrO- |
| √) HPO3 Ц H4P2O7 Ц H3PO4 | ак и в случае (б), в данном р€ду количество атомов кислорода, св€занных только с центральным атомом слева направо уменьшаетс€, следовательно уменьшаетс€ сила кислот. H3PO4 = 3H+ + –ќ43- |
604. „ем слабее св€зь ћеЧќЌ в основании, тем выше сила основани€. “.е., чем больше радиус атома металла, чем меньше степень окислени€ металла, чем меньше пол€ризующа€ способность катиона металла, тем сильнее соответствующее основание. “.о., в р€ду LiOH-Е-CsOH сила оснований увеличиваетс€ (растет радиус металла), Fe(OH)2 сильнее, чем Fe(OH)3 (степень окислени€ железа +2 и +3), Ca(OH)2 сильнее, чем Zn(OH)2 (пол€ризующа€ способность кальци€ меньше, чем цинка).
|
|
|
605. 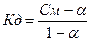 , поскольку величина константы диссоциации небольша€, то степенью диссоциации - а по сравнению с 1 в знаменателе можно пренебречь, тогда
, поскольку величина константы диссоциации небольша€, то степенью диссоциации - а по сравнению с 1 в знаменателе можно пренебречь, тогда 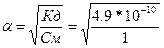 = 2.2*10-5.
= 2.2*10-5.
606.
| ƒано: a1 = 0.42 %, —1= 1ћ a2 = 1.33 %, —2= 0.1ћ a3 = 4.22%, —3= 0.01ћ д -? | 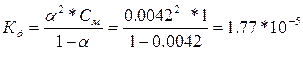
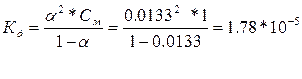
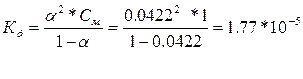 — увеличением разбавлени€ возрастает степень диссоциации, а константа диссоциации остаетс€ неизменной, т.е. она не зависит от концентрации раствора.
— увеличением разбавлени€ возрастает степень диссоциации, а константа диссоциации остаетс€ неизменной, т.е. она не зависит от концентрации раствора.
|
607.
| ƒано: a = 0.1 % —м= 1ћ. д -? | 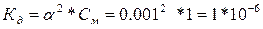 ѕо справочным данным данна€ д относитс€ к NH4OH. ѕо справочным данным данна€ д относитс€ к NH4OH.
|
608.
| ƒано: д1 = 1.3*10-2 д2 = 0.6*10-7 —м= 0.1м | ј) H2S = H+ +HS- 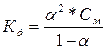 = 1.3*10-2 = = 1.3*10-2 = 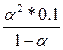 , следовательно, α1=0.3=30%
—м(HS-) = α1*—м(H2S) = 0.3*0.1 = 0.03 моль/л
Ѕ) HS- = H+ + S2- = , следовательно, α1=0.3=30%
—м(HS-) = α1*—м(H2S) = 0.3*0.1 = 0.03 моль/л
Ѕ) HS- = H+ + S2- = 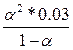 = 0.6*10-7, следовательно, α2 = 0.0014 = 0.14% = 0.6*10-7, следовательно, α2 = 0.0014 = 0.14%
|
609.
| ƒано: д = 1.8*10-5 —м1 = 0.1 м —м2 = 0.001 м | «акон ќствальда: 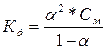 ј) 1.8*10-5 = ј) 1.8*10-5 = 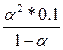 , a1 = 0.0134
Ѕ) 1.8*10-5 = , a1 = 0.0134
Ѕ) 1.8*10-5 = 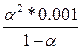 , a2 = 0.134 a2/a1 = 10 раз , a2 = 0.134 a2/a1 = 10 раз
|
610. ¬ растворе сильные электролиты диссоциируют нацело, образу€ ионы. “.к. молекулы воды пол€рны, то они окружают ионы электролита, что приводит к кажущемус€ уменьшению концентрации и степени диссоциации электролита.
| ƒано: Cm(KCl) = 1моль/кг “(зам) = -3.360— i, a(каж) =? | 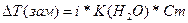 = i*1.86*1 = 3.36, следовательно, i = 1.8
a(каж) = (i-1)/(m-1) = (1.8-1)/(2-1) = 0.8 = 80% = i*1.86*1 = 3.36, следовательно, i = 1.8
a(каж) = (i-1)/(m-1) = (1.8-1)/(2-1) = 0.8 = 80%
|
611. ¬ растворе сильные электролиты диссоциируют нацело, образу€ ионы. “.к. молекулы воды пол€рны, то они окружают ионы электролита, что приводит к кажущемус€ уменьшению концентрации и степени диссоциации электролита. “.к. раствор глюкозы —м = 0.44м изотоничен раствору NaCl —н = 0.25м, то изотонический коэффициент i = 0.44/0.25 = 1.76. —тепень диссоциации 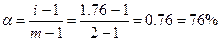 , где m Ц число ионов, на которые диссоциирует электролит.
, где m Ц число ионов, на которые диссоциирует электролит.
612. ¬ растворе сильные электролиты диссоциируют нацело, образу€ ионы. “.к. молекулы воды пол€рны, то они окружают ионы электролита, что приводит к кажущемус€ уменьшению концентрации и степени диссоциации электролита.
| ƒано: m(NH4Cl) = 1.07 г V(H2O) = 200 мл. “(кип) = 100.09 0— a(каж) =? | 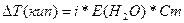 . Ќайдем мол€льность раствора —m = =n(NH4Cl)/m(H2O) = . Ќайдем мол€льность раствора —m = =n(NH4Cl)/m(H2O) = 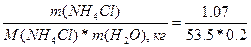 = 0.1 моль/кг
0.09 = i*0.52*0.1 => i = 1.73
a(каж) = (i-1)/(m-1) = (1.73-1)/(2-1) = 0.73 = 73%, где m Ц количество ионов, на которые диссоциирует электролит. = 0.1 моль/кг
0.09 = i*0.52*0.1 => i = 1.73
a(каж) = (i-1)/(m-1) = (1.73-1)/(2-1) = 0.73 = 73%, где m Ц количество ионов, на которые диссоциирует электролит.
|
613. ¬ растворе сильные электролиты диссоциируют нацело, образу€ ионы. “.к. молекулы воды пол€рны, то они окружают ионы электролита, что приводит к кажущемус€ уменьшению концентрации и степени диссоциации электролита.
| ƒано: –0 = 101.3 кѕа V(H2O) = 450 мл n(Na2SO4) = 0.05моль PТ = 100.8 кѕа a(каж) =? | Po - PТ = i*P0*N(Na2SO4)
n(H2O) = m/M = 450/18 = 25 моль.
101.3 Ц 100.8 = 101.3*i* 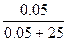 , следовательно, i = 2.47
a(каж) = (i-1)/(m-1) = (2.47-1)/(3-1) = 0.73 = 73 %, где m Ц количество ионов, на которые диссоциирует электролит. , следовательно, i = 2.47
a(каж) = (i-1)/(m-1) = (2.47-1)/(3-1) = 0.73 = 73 %, где m Ц количество ионов, на которые диссоциирует электролит.
|
614.
| ƒано: Cм(’) = 0.04моль/л “ = 00— = 273 Pосм = 217.8 кѕа ј = 70% m =? | Pосм = i—м(’)*R*T = i*0.04*8.31*273 = 217.8 кѕа i = 2.4 —тепень диссоциации а = (i-1)/(m-1) = 0.70, следовательно, m = 3. ¬ещество диссоциирует на три иона. ѕримеры электролитов: CaCl2, H2SO4 и т.д. |
615. “еоретически i=2,a=100 %, “зам.= -3.720 —, практически “зам.= -3.360 —, m = 1моль/кг.
¬ растворе KCl диссоциирует на ионы: KCl = K+ + Cl-. ѕол€рные молекулы воды окружают эти ионы, что приводит к кажущемус€ изменению концентрации. a(практич.) = (3.36/3.72)*100% = 90%. i=1 + (n-1)* a = 1+(2-1)*0.9 = 1.9
|
|
|
616.
| Na2[Sn(OH)6] = 2Na+ + [Sn(OH)6]2- | д = 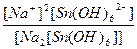
| н = 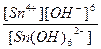
|
| [Cr(NH3)6](NO3)3 = [Cr(NH3)6]3+ + 3NO3- | д = 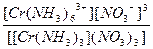
| н = 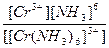
|
617.
| K2[NiCl4] = 2K+ + [NiCl4]2- | д = 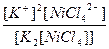
| н = 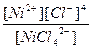
|
| [PtF2(NH3)4](NO3)2 = [PtF2(NH3)4]2+ + 2NO3- | д = 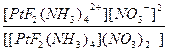
| н = 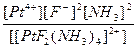
|
618.
| (NH4)2[RuCl6] = 2NH4+ + [RuCl6]2- | д = 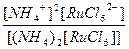
| н = 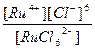
|
| [Ag(NH3)2]Cl = [Ag(NH3)2]+ + Cl- | д = 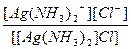
| н = 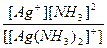
|
619.
| Na2[Ni(CN)4] = 2Na+ + [Ni(CN)4]2- | д = 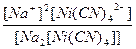
| н = 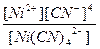
|
| [PtCl2(NH3)4]SO4 = [PtCl2(NH3)4]2+ + SO42- | д = 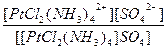
| н = 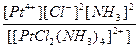
|
620.
| K3[CoF6] = 3K+ + [CoF6]3- | д = 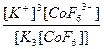
| н = 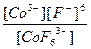
|
| [Fe(NH3)6](NO3)2 = [Fe(NH3)6]2+ + 2NO3- | д = 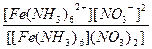
| н = 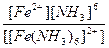
|






