» электродвижущие силы
ѕримеры решени€ задач
«адача 1. Ёƒ— гальванического элемента
Zn | ZnSO4 (0,1 M) || KClнас, Hg2Cl2 | Hg
при 25 ∞— равна 1,03 ¬. Ќапишите уравнени€ электродных реакций и суммарную реакцию, протекающую в элементе. ќпределите электродный потенциал цинкового электрода в этом растворе и сравните его с теоретически рассчитанным по формуле Ќернста.
–ешение
ѕриведем справочные значени€ стандартных электродных потенциалов:  , в каломельном электроде дл€ насыщенного раствора —l
, в каломельном электроде дл€ насыщенного раствора —l  . “ак как стандартный потенциал цинкового электрода имеет меньшее значение по сравнению со стандартным потенциалом каломельного электрода, на цинковом электроде протекает реакци€ окислени€:
. “ак как стандартный потенциал цинкового электрода имеет меньшее значение по сравнению со стандартным потенциалом каломельного электрода, на цинковом электроде протекает реакци€ окислени€:
Zn∞ Ц 2ē = Zn2+,
а на каломельном Ч реакци€ восстановлени€:
Hg2Cl2 + 2ē = 2Hg∞ + 2ClЦ.
—уммарное уравнение реакции имеет вид
Zn∞ + Hg2Cl2 = Zn2+ + 2Hg∞ + 2ClЦ.
Ёƒ— цепи равна разности потенциалов положительного и отрицательного электродов:  . ќтсюда выразим потенциал цинкового электрода и рассчитаем его значение:
. ќтсюда выразим потенциал цинкового электрода и рассчитаем его значение:
 .
.
“еоретическое значение электродного потенциала вычислим по формуле Ќернста:
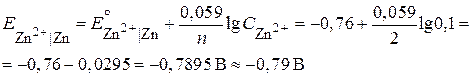
«адача 2. Ёƒ— хингидронно Ц каломельного гальванического элемента при 25 ∞— равна 0,094 ¬. ’ингидронный электрод опущен в фосфатную буферную смесь. ќпределите рЌ и концентрацию ионов водорода буферной смеси, если стандартный электродный потенциал хингидронного электрода равен 0,699 ¬, а потенциал насыщенного каломельного электрода равен 0,24 ¬.
–ешение
—равним стандартные электродные потенциалы хингидронного и каломельного электродов. ѕоскольку стандартный потенциал хингидронного электрода более положительный, в схеме гальванического элемента запишем его справа: Hg | Hg2Cl2 , KClнас || H+, хг | –t.
¬ данной цепи на хингидронном электроде протекает процесс восстановлени€: —6Ќ4ќ2 + 2Ќ+ + 2ē = —6Ќ4(ќЌ)2,
хинон гидрохинон
а на каломельном электроде - процесс окислени€: 2Hg∞ + 2ClЦЦ 2ē = Hg2Cl2. Ёƒ— цепи равна разности потенциалов: 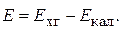
ѕотенциал хингидронного электрода зависит от активности ионов водорода ( ) в растворе:
) в растворе:  , где
, где  - стандартный электродный потенциал.
- стандартный электродный потенциал.
ƒл€ разбавленных растворов  ї
ї 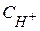 , следовательно
, следовательно
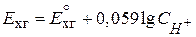 ,
,
где 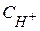 - мол€рна€ концентраци€ ионов водорода.
- мол€рна€ концентраци€ ионов водорода.
“аким образом, выражение дл€ расчета Ёƒ— примет вид:
 ,
,
–ассчитаем концентрацию ионов водорода:
 ,
,
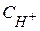 = 10‑6,186 = 10‑7×100,814 = 6,5×10Ц7 моль/л,
= 10‑6,186 = 10‑7×100,814 = 6,5×10Ц7 моль/л,

«адача 3. ћожет ли работать гальванический элемент за счет протекани€ реакции: Fe2+ + Co3+ = Fe3+ + Co2+?
—оставьте схему и рассчитайте стандартное значение Ёƒ— этого элемента.
–ешение
—равним справочные значени€ стандартных окислительно-восстановительных электродных потенциалов:


“ак как  , окисленна€ форма второго электрода (—о3+) будет служить окислителем дл€ восстановленной формы первого электрода (Fe2+), т. е. при составлении гальванической цепи из данных электродов будет осуществл€тьс€ следующа€ реакци€: Fe2+ + Co3+ = Fe3+ + Co2+ .
, окисленна€ форма второго электрода (—о3+) будет служить окислителем дл€ восстановленной формы первого электрода (Fe2+), т. е. при составлении гальванической цепи из данных электродов будет осуществл€тьс€ следующа€ реакци€: Fe2+ + Co3+ = Fe3+ + Co2+ .
Ёлектрод, стандартный окислительно-восстановительный потенциал которого имеет большее значение, располагаем справа. “аким образом схема гальванического элемента имеет вид:
|
|
|
–t | Fe3+, Fe2+ || Co3+, Co2+ | –t
—тандартное значение Ёƒ— рассчитываем по формуле
 .
.
¬опросы и задани€
1. ќбъ€сните механизмы возникновени€ скачков потенциала на границах раздела: а) металл - раствор соли этого металла; б) раствор —uSќ4 - раствор ZnSO4.
2. акой процесс (окисление или восстановление) протекает при работе гальванического элемента на левом электроде?
3. акие процессы могут привести к возникновению скачка потенциала на границе раздела металл - раствор соли этого металла?
4. — какой целью при составлении гальванических элементов примен€ют солевой мостик, заполненный —l?
5. ¬ каком растворителе и почему потенциал цинковой пластинки будет более отрицательным: а) вода; б) бензол; в) этанол?
6. ѕри каких услови€х потенциал электрода будет стандартным?
7. ѕри погружении цинка в раствор сульфата меди происходит реакци€, аналогична€ той, что протекает при работе гальванического элемента якоби Ц ƒаниэл€. ¬ какие виды энергии превращаетс€ в каждом случае энерги€ химической реакции?
8. ак (положительно или отрицательно) будет зар€жена медна€ плас≠тинка в 1 ћ растворе CuSO4?
9. Ѕудет ли зар€жена (и как) пластинка из никел€ в чистой воде и 1 ћ растворе NiSO4?
10. аким образом можно осуществить превращение энергии химической реакции в электрическую?
11. ак вли€ет температура и активность ионов Zn2+ на величину электродного потенциала цинковой пластинки?
12. акие процессы могут привести к возникновению скачка потенциала на границе раздела раствор(I) - раствор(II). ћожно ли измерить величину этого скачка потенциала?
13. ћожно ли хингидронный электрод отнести к окислительноЦвосстановительным? акова роль металла в этом случае?
14. Ќапишите уравнени€ химических реакций, на основе которых работает гальванический элемент: Ni | Ni2+ || 2H+, H2 | –t.
15. ƒл€ определени€ рH раствора электрометрическим методом составлен гальванический элемент из хлорсеребр€ного и хингидронного электродов. акой электрод выступает в роли электрода сравнени€? »зобразите схему гальванического элемента. аким электродом можно заменить: а) хлорсеребр€ный электрод; б) хингидронный электрод.
16. —тандартные потенциалы цинкового и серебр€ного электродов при 298 равны соответственно Ц0,763 и +0,799 ¬. акую информацию можно получить из сопоставлени€ этих величин? ак экспериментально измер€ют стандартные потенциалы электродов?
17. —тандартный потенциал никелевого электрода при 298 равен
Ц0,25 ¬. ак измер€ют значени€ стандартных потенциалов и почему их называют условными? ак будет зар€жена поверхность никел€ в 1 ћ растворе сульфата никел€ (положительно или отрицательно)?
18. „ем отличаетс€ по механизму окислительно-восстановительна€ реакци€, протекающа€ при работе гальванического элемента, от "обычной"?
19. ѕредложите методы определени€ активности ионов водорода. — какой целью было введено пон€тие рH раствора?
20. ќбъ€сните механизм возникновени€ и роль мембранного потенциала.
21. ќбъ€сните преимущества электрометрического метода измерени€ рH биологических жидкостей.
22. ѕредложите метод определени€ отношени€ окисленной и восстановленной форм вещества в растворе электрохимическим методом.
23. Ѕудет ли зар€жена (и как) цинкова€ пластинка: а) в чистой воде; б) 0,1 ћ растворе ZnSO4; в) бензоле?
|
|
|
24. ћожно ли определить рH биологических жидкостей методом титровани€?
25. — какой целью при измерении Ёƒ— элементов используют солевой мостик, элемент ¬естона?
26. ћожно ли измерить величину скачка потенциала на границе металл - раствор?
27. акие электроды называютс€ окислительно-восстановительными? — какой целью при их изготовлении используют инертные металлы?
28. ќбъ€сните механизм возникновени€ диффузионного потенциала. аким способом его можно устранить?
29. „то представл€ет собой стандартный водородный электрод? ћожет ли он выступать в гальваническом элементе в роли положительного электрода?
30. ќбъ€сните сущность потенциометрических методов определени€ рЌ биологических жидкостей.
31. ак (положительно или отрицательно) будет зар€жена цинкова€ пластинка в 0,1 ћ растворе ZnSO4?
32. √альванический элемент был составлен из каломельного и хлорсеребр€ного электродов. ћожно ли использовать данную систему дл€ измерени€ рH желудочного сока?
33. ћожно ли определить возможность и направление окислительного процесса по величине редоксЦпотенциала?
34. ѕредложите наиболее простой метод определени€ рH желудочного сока.
35. Hе прибега€ к расчетам, сопоставьте значени€ Ёƒ— двух элементов:
Zn | ZnSO4 (0,1 ћ) || ZnSO4 (1 ћ) | Zn
Cu | CuSO4 (0,01 ћ) || CuSO4 (0,1ћ) | Cu.
36. Ѕудет ли работать гальванический элемент, составленный из медного и серебр€ного электродов, погруженных в 1 ћ растворы нитратов этих металлов? акой электрод будет положительным?
37. — какой целью при измерении Ёƒ— гальванических элементов примен€ют нормальный элемент ¬естона?
38. акие из приведенных электродов можно примен€ть в качестве электродов сравнени€:
ZnSO4 | Zn; KCl (насыщ), AgCl | Ag; Fe2+, Fe3+ | Pt; H+, H2 | Pt;
KCl (насыщ), Hg2Cl2 | Hg; H+, C6H4O2, C6H4(OH)2 | Pt.
39. акой потенциал называют диффузионным? ќбъ€сните механизм его возникновени€. ”читывают ли его величину при расчетах Ёƒ— элементов?
40. акой гальванический элемент называют концентрационным?
41. ћожно ли водородный электрод использовать в качестве: а) электрода сравнени€; б) индикаторного электрода при измерении рH растворов.
42. »спользу€ справочные значени€ стандартных электродных потенциалов алюмини€ и серебра, сделайте вывод о том, в какой посуде лучше хранить воду: в алюминиевой или серебр€ной.
43. ћожно ли измерить потенциал одного электрода?
44. аковы преимущества стекл€нного электрода перед другими электродами, примен€емыми дл€ той же цели?
45. акие электроды примен€ютс€ при определении рH растворов в качестве индикаторных? ѕриведите уравнени€, отражающие вли€ние рH на величину электродного потенциала индикаторных электродов.
46. ѕредложите метод определени€ ионов серебра в родниковой воде, использу€ каломельный электрод и серебр€ную пластинку.
47. ћожет ли Ёƒ— элемента быть отрицательной?
48. акие электроды можно использовать в качестве электродов сравнени€?
49. аково термодинамическое условие образовани€ положительного зар€да на поверхности металла, погруженного в раствор соли этого металла?
50. ќбъ€сните услови€ возникновени€ отрицательного зар€да на поверхности металла, который опущен в раствор своей соли.
51. ќбъ€сните причины возникновени€ диффузионного потенциала. аким образом устран€ют его вли€ние?
52. —уществуют ли гальванические элементы, дл€ которых величина Ёƒ— не зависит от величины стандартных электродных потенциалов? ≈сли существуют, то укажите тип этих элементов и приведите примеры.
53. Ёƒ— цепи, составленной из насыщенного каломельного электрода и хингидронного электрода в исследуемом растворе, равна 0,274 ¬ при 298 . –ассчитайте рH исследуемого раствора. ѕотенциал насыщенного каломельного электрода равен 0,24 ¬. (3,14)
54. »зобразите схему гальванического элемента, составленного из цинкового и никелевого электродов, погруженных в 0,2 ћ растворы сульфатов этих металлов. –ассчитайте Ёƒ— этого элемента при 298 . —тандартные потенциалы никелевого и цинкового электродов равны Ц0,25 ¬ и Ц0,76 ¬ соответственно. (0,52 ¬)
|
|
|
55. ѕравильно ли экспериментально определены потенциалы цинкового электрода в 0,01 ћ и 1 ћ растворах, если измеренные значени€ Ёƒ— элемента, составленного из хлорсеребр€ного и цинкового электродов, равны 1,12 и 1,25 ¬?
56. Ёлектродный потенциал серебра в 0,2 ћ растворе нитрата серебра равен 0,747 ¬. ¬ычислите величину стандартного потенциала серебр€ного электрода при 298 , счита€ коэффициент активности ионов равным 0,66. (0,799 ¬)
57. »зобразите схему гальванического элемента, составленного из двух водородных электродов, заполненных 1 ћ и 0,1 ћ растворами сол€ной кислоты. акова Ёƒ— такого элемента? какому типу его можно отнести?
58. Ќапишите в ионной форме уравнение реакции, протекающей при работе никелевоЦцинкового элемента. акой электрод будет отрицательным? –ассчитайте Ёƒ— данного элемента при концентрации растворов солей равной 0,1 ћ и температуре 298 . —тандартные потенциалы никелевого и цинкового электродов равны Ц0,25 ¬ и Ц0,76 ¬ соответственно. (0,51 ¬)
59. ѕри измерении рH желудочного сока пациентов был использован гальванический элемент, состо€щий из хингидронного и насыщенного каломельного электродов. ќцените значени€ Ёƒ— этого элемента в случае пониженной и повышенной кислотности, если рЌ желудочного сока здорового человека считать равным 1,1. —тандартный потенциал хингидронного электрода 0,7 ¬, потенциал насыщенного каломельного электрода 0,24 ¬.
60. –ассчитайте рH раствора, если потенциал хингидронного электрода в нем при 298 равен 0,514 ¬. (3,15)
61. –ассчитайте потенциал хингидронного электрода в растворе с рH=3, а также Ёƒ— хингидронно Ц каломельного элемента. ѕотенциал каломельного электрода в 1 н растворе KCl равен 0,28 ¬. (0,523 ¬; 0,243 ¬)
62. –ассчитайте потенциал хингидронного электрода, рH раствора, если Ёƒ— хингидронно Ц каломельного элемента при 298 равна 0,274 ¬. ѕотенциал насыщенного каломельного электрода равен 0,24 ¬. (0,514 ¬; 3,15)
63. —тандартный электродный потенциал никелевого электрода равен
Ц0,25 ¬. –ассчитайте величину электродного потенциала никелевого электрода в 0,01 ћ растворе NiSO4. (Ц0,309 ¬)
64. –ассчитайте потенциал водородного электрода в растворе Ќ—l с рЌ=3. «апишите схему гальванического элемента, составленного из данного водородного электрода и хлорсеребр€ного электрода, и уравнени€ электродных процессов. (Ц0,177 ¬)
65. –ассчитайте потенциал водородного электрода в желудочном соке, если концентраци€ ионов водорода равна 0,08 моль/л. ѕриведите условную форму записи данного электрода и уравнение электродного процесса, если данный электрод выполн€ет функцию отрицательного электрода. (Ц0,065 ¬)
66. –ассчитайте величину потенциала электрода –t | хингидрон, HCl (0,1 ћ). какому типу относитс€ этот электрод? ѕриведите уравнение электродного процесса, если хингидронный электрод выполн€ет функцию положительного электрода. (0,641 ¬)
67. ¬ычислите потенциал водородного электрода, погруженного в чистую воду, раствор с рЌ=3,5, раствор с рЌ=10,7. (Ц0,413 ¬; Ц0,21 ¬; Ц0,63 ¬)
68. ¬ычислите Ёƒ— гальванического элемента, если железный и медный электроды опущены в растворы их солей одинаковой концентрации. Ќапишите уравнение реакции, котора€ €вл€етс€ источником электронов. —тандартные потенциалы железного и медного электродов равны Ц0,44 ¬ и 0,34 ¬ соответственно.
69. ƒл€ измерени€ рЌ желчи был составлен гальванический элемент из водородного и хлорсеребр€ного электродов. Ёƒ— этого элемента при 298 и концентрации электролита в электроде сравнени€ равной 1 моль/л оказалась равной 0,577 ¬. –ассчитайте рЌ желчи. (5,75)
|
|
|
70. ƒл€ измерени€ рH крови был собран хингидронно Ц каломельный элемент. »зобразите схему этого элемента. ѕравильно ли были проведены измерени€, если Ёƒ— оказалась равной 0,126 ¬? ѕотенциал каломельного электрода равен 0,24 ¬, а стандартный потенциал хингидронного электрода 0,70 ¬; рЌ сыворотки крови в норме имеет значение 7,4 ± 0,05. (0,0234 ¬)
71. ¬ычислите активность ионов никел€ в растворе сульфата никел€, если потенциал никелевого электрода в этом растворе равен Ц0,255 ¬. (0,677 моль/л)
72. »зобразите схему гальванического элемента, составленного из двух серебр€ных электродов, погруженных в 0,1 ћ и 1 ћ растворы нитрата серебра. Ѕудет ли работать такой элемент? какому типу его можно отнести? аково значение Ёƒ—? (0,059 ¬)
73. »зобразите схему гальванического элемента, составленного из никелевой и кадмиевой пластинок, погруженных в 1 ћ растворы сульфатов этих металлов. ака€ реакци€ будет протекать при работе этого элемента? –ассчитайте величину Ёƒ— элемента при 298 . (0,62 ¬)
74. ќбъ€сните принцип компенсационного метода измерени€ Ёƒ—. акова роль элемента ¬естона в этой схеме?
75. ћожет ли работать элемент за счет протекани€ реакции:
2Fe3+ + Sn2+ = 2Fe2+ + Sn4+.
–ассчитайте стандартное значение Ёƒ— этого элемента при 298 . акие металлы погружены в растворы?
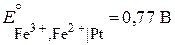 ,
, 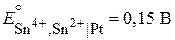 .
.
76. Ёƒ— элемента, составленного из водородного и хлорсеребр€ного электродов при 298 , имеет значение 0,303 ¬? ѕотенциал хлорсеребр€ного электрода при этой температуре равен 0,222 ¬. —ледует ли бить тревогу пациенту, если его желудочный сок был использован в качестве исследуемого раствора в водородном электроде? (1,37)
77. »зобразите схему элемента, при работе которого будет протекать реакци€ Zn0 + Ni2+= Zn2+ + Ni0. –ассчитайте Ёƒ— данного элемента, если концентраци€ сульфата цинка составл€ет 0,1 моль/л, а сульфата никел€ - 1 моль/л. —тандартный потенциал никелевого и цинкового электродов равны Ц0,25 ¬ и Ц0,76 ¬ соответственно. (0,55 ¬)
78. ака€ реакци€ будет протекать при работе гальванического элемента, составленного из цинкового и никелевого электродов, погруженных в 0,1 ћ растворы сульфатов этих металлов? »зобразите схему элемента и рассчитайте величину Ёƒ— при 298 . —тандартный потенциал никелевого и цинкового электродов равны Ц0,25 ¬ и Ц0,76 ¬ соответственно. (0,51 ¬)
79. ѕотенциал кадмиевого электрода в 0,1 ћ растворе сульфата кадми€ при 298 равен Ц0,441 ¬. —редний коэффициент активности ионов кадми€ имеет значение 0,516. ¬ычислите значение стандартного потенциала кадмиевого электрода. (Ц0,403 ¬)
80. ƒл€ определени€ рH была измерена Ёƒ— элемента, составленного из двух водородных электродов: стандартного и заполненного желудочным соком. ѕонижена или повышена кислотность сока, если Ёƒ— этого элемента при 298 равна 0,085 ¬? (1,44)
81. акое значение рЌ имеет раствор кислоты в стандартном водородном электроде, потенциал которого прин€т за 0? ак изменитс€ потенциал при рЌ равном 2? (0; Ц0,118 ¬)
82. »зменитс€ ли величина электродного потенциала меди в растворе CuSO4, если раствор разбавить в 10 раз? —тандартный электродный потенциал медного электрода равен 0,34 ¬.
83. —оставьте схему гальванического элемента, име€ в распор€жении Sn, Ag, SnCl2 и AgNO3. Ќапишите уравнение реакции, протекающей при работе данного элемента.
84. ѕотенциал водородного электрода, заполненного желудочным соком, при 298 равен Ц0,082 ¬. —ледует ли пациенту беспокоитьс€ о своем здоровье?
85. –ассчитайте Ёƒ— гальванического элемента при 298 :
Pt | Fe3+, Fe2+ || Co3+, Co2+ | Pt,
если концентрации солей двухвалентных металлов равны 0,2 моль/л, а солей трехвалентных металлов Ц 0,4 моль/л.
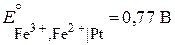 ,
, 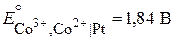 . (1,07 ¬)
. (1,07 ¬)
86. √альванический элемент составлен из стандартного водородного электрода и водородного электрода, помещенного в раствор кислоты с рЌ=6. –ассчитайте Ёƒ— данного гальванического элемента и представьте условную форму его записи. (0,354 ¬)
87. –ассчитайте электродный потенциал магниевого электрода в растворе Mg(NO3)2 с концентрацией 0,03 моль/л, если степень диссоциации соли равна 60%. —тандартный электродный потенциал магниевого электрода равен Ц2,36 ¬. (Ц2,41 ¬)
88. Ёƒ— цепи, составленной из насыщенного каломельного электрода и водородного электрода, опущенного в исследуемой раствор спинномозговой жидкости, равна 0,678 ¬ при 25 ∞—. –ассчитайте рЌ спинномозговой жидкости, если потенциал насыщенного каломельного электрода равен 0,244 ¬. (7,36)
89. ¬ычислить активность ионов водорода в растворе, в котором потенциал водородного электрода равен Ц0,082 ¬, “ = 298 . (0,04 моль/л)
90. ƒл€ определени€ рЌ желчи (из желчного пузыр€) была составлена цепь из водородного и хлорсеребр€ного электродов, Ёƒ— которой оказалась равной 0,577 ¬ при температуре 298 . онцентраци€ электролита в электроде сравнени€ равна 1 моль/л. ќпределите рЌ желчи и сравните его со значением 5,75, что соответствует норме.
|
|
|
| ¬арианты индивидуальных заданий дл€ контрольной работы по теме: У‘изико-хими€ поверхностных €вленийФ | Ќомера вопросов | ѕоследн€€ цифра | 10, 26, 41, 67, 70 | 4, 21, 38, 49, 88 | 14, 31, 57, 69,71 | 13, 22, 34, 56, 87 | 5, 38, 43, 65, 85 | 6, 27, 33, 63, 83 | 8, 25, 26, 62, 64 | 2, 16, 19, 54,58 | 21, 23, 37, 59, 86 | 18, 34, 50, 80, 81 | |
| 9, 25, 39, 66, 69 | 3, 20, 37, 86, 87 | 13, 28, 46, 65, 68 | 14, 21, 30, 55, 84 | 6, 35, 42, 64, 82 | 5, 19, 57, 61, 80 | 5, 30, 36, 54, 70 | 1, 18,34, 56, 88 | 17, 34, 39, 52, 85 | 12, 15, 40, 67, 83 | ||||
| 8, 24, 40, 63, 65 | 2, 19, 34, 84, 85 | 12, 27, 43, 64, 67 | 9, 20, 49 57, 83 | 16, 30,36, 62, 81 | 3, 18, 24, 58, 74 | 20, 23, 37, 60,86 | 21, 30, 53, 55, 80 | 16, 31, 35, 51, 82 | 11, 36, 41, 68, 78 | ||||
| 7, 23, 36, 61, 64 | 1, 18, 53, 82, 83 | 11, 30, 45, 54, 66 | 6, 19, 45, 74, 79 | 9, 33, 37, 60, 71 | 2, 17, 20, 56, 80 | 24, 32, 34, 59, 85 | 12, 15, 49, 50, 87 | 15, 29, 42, 48, 81 | 21, 31, 38, 63, 77 | ||||
| 6, 22, 38, 58, 62 | 16, 32, 57, 79, 81 | 10, 29, 44, 60, 63 | 5, 18, 26, 76, 80 | 3, 25, 34, 59, 68 | 1, 4, 12, 55, 78 | 10, 17, 31, 52, 82 | 8, 11, 41, 77, 84 | 4, 14, 36, 71, 75 | 7, 39, 57, 66, 72 | ||||
| 5, 21, 37, 56, 60 | 15, 31, 46, 76, 80 | 9, 26, 42, 59, 61 | 3, 4, 12, 75, 78 | 8, 21,24, 52, 67 | 11,14, 49, 53, 77 | 8, 16, 34, 51, 81 | 7, 10, 40, 79, 83 | 10, 13, 28, 68, 73 | 35, 45, 47, 58, 70 | ||||
| 4, 20, 34, 55, 59 | 14, 28, 43, 75, 78 | 8, 25, 41, 58, 62 | 2, 10,11, 77, 86 | 7, 23, 31, 51,66 | 7, 38, 50, 73, 79 | 15, 29, 48, 57, 71 | 4, 39,46, 72, 76 | 8, 9, 32, 67, 74 | 26, 33, 44, 61,69 | ||||
| 3, 19, 52, 53, 54 | 13, 27, 45, 74, 77 | 7, 24, 39, 52, 56 | 1, 7, 8, 73, 85 | 17, 29, 42, 48, 63 | 35, 40, 47, 72, 76 | 14, 28, 45, 68, 88 | 26, 38, 44, 75,78 | 6, 22, 33, 66, 79 | 4, 30, 43, 55,65 | ||||
| 2, 18, 35, 49, 51 | 12, 30, 44, 71, 73 | 6, 23, 40, 48, 55 | 16, 17, 35, 72, 82 | 15, 28, 30, 54, 61 | 6, 41, 46, 70, 75 | 12, 22, 26, 67, 87 | 33,45,47, 69, 74 | 5, 27, 38, 63, 76 | 10, 25, 42, 56, 64 | ||||
| 1, 17, 33, 48, 76 | 11, 29, 42, 68, 72 | 5, 22, 36, 51, 54 | 15, 32, 33, 70, 81 | 10, 16, 27, 58, 88 | 21, 39, 44, 69, 86 | 4, 9, 21, 66, 84 | 35, 43, 57, 65, 73 | 3, 20, 26, 61, 62 | 8, 24, 46, 49, 60 | ||||
| ѕред- | послед-н€€ | цифра |






