»ндивидуальные
«адани€ по физической
» коллоидной химии
ѕрактикум
»здание второе, исправленное
и дополненное
—аранск
»здательство ћордовского университета
”ƒ 541.1+541.18 (076.1)
ЅЅ √
»60
јвторы: Ћ. ј. ∆ивечкова, Ё. ¬. –оманова, ≈. ѕ. оновалова, Ќ. ѕ. —ыркина
»ндивидуальные задани€ по физической и коллоидной химии: / Ћ.ј. ∆ивечкова, ≈.ѕ. оновалова, Ё.¬. –оманова, Ќ.ѕ. —ыркина - »зд. 2-е, испр. и доп.Ц —аранск: »зд-во ћордов. ун-та, 2009. - 79 с.
Ќасто€щие материалы содержат индивидуальные задани€ по физической и коллоидной химии и примеры решени€ задач, сгруппированные по основным модул€м курса. ѕриводитс€ список использованной литературы.
ѕредназначено дл€ студентов нехимических специальностей.
ѕодписано в печать 30.07.09. ‘ормат 60 ´ 841/16.
”сл. печ. л. 4,42. “ираж 300 экз. «аказ є1029
»здательство ћордовского университета
“ипографи€ »здательства ћордовского университета
430005 —аранск, ул. —оветска€, 24
ѕ–≈ƒ»—Ћќ¬»≈
¬ насто€щее врем€ профессиональна€ де€тельность медика и биолога требует глубоких знаний в области химии, овладени€ современными химическими и физико-химическими методами исследовани€. ѕри этом особую роль приобретает изучение физической и коллоидной химии. ѕонимание физико-химических закономерностей функционировани€ живого организма послужит фундаментом дл€ последующего изучени€ специальных дисциплин, таких, как биологическа€ хими€, биотехнологи€, физиологи€, фармакологи€ и др.
÷ель насто€щего практикума - оказать помощь студентам медицинских и биологических специальностей в самосто€тельной работе при изучении физической и коллоидной химии, сформировать умение использовать теоретические знани€ в будущей практической и профессиональной де€тельности.
ѕредлагаемые вопросы и задачи сгруппированы по основным темам данного курса и, насколько возможно, нос€т медико-биологический характер. ¬ начале каждого раздела приведены примеры решени€ типовых задач. ƒл€ установлени€ варианта к каждому разделу приводитс€ таблица, в которой по горизонтали берут предпоследнюю цифру варианта, а по вертикали - последнюю. ѕоливариантность заданий предполагает строгую индивидуальность при выполнении студентами контрольных работ. ¬ задачах, требующих решени€, в скобках указаны ответы.
| ¬арианты индивидуальных заданий дл€ контрольной работы по теме: У’имическа€ термодинамикаФ | Ќомера вопросов | ѕоследн€€ цифра | 10, 26, 42, 74, 81 | 4, 21, 38, 55, 72 | 14, 31, 48, 49, 86 | 12, 27, 42, 57, 72 | 2, 19, 34, 66, 84 | 12, 29, 44, 61, 88 | 6, 24, 42, 60, 78 | 16, 18, 36, 54, 72 | 2, 32, 46, 60, 74 | 11, 28, 45, 60,75 | |
| 9, 25, 41, 57, 88 | 3, 20, 37, 61, 82 | 13, 30, 47, 64, 87 | 11, 26, 41, 56, 86 | 1, 18, 33, 50, 65 | 11, 28, 60, 73, 75 | 5, 23, 41, 59, 77 | 15, 17, 35, 81, 85 | 1, 31, 45, 59, 76 | 12, 29, 46, 61, 76 | ||||
| 8, 24, 40, 56, 72 | 2, 3, 19, 55, 70 | 12, 29, 46, 63, 80 | 10, 25, 40, 55, 70 | 2, 19, 34, 80, 83 | 10, 27, 42, 59, 74 | 4, 22, 40, 82, 76 | 14, 32, 34, 52, 84 | 16, 30, 44, 72, 82 | 13, 30, 47, 62, 77 | ||||
| 7, 23, 39, 55, 86 | 1, 18, 33, 35, 69 | 11, 28, 45, 62, 79 | 9, 24, 39, 54, 74 | 3, 18, 39, 64, 79 | 9, 14, 26, 41, 87 | 3, 21, 39, 57, 75 | 13, 31, 33, 51, 69 | 15, 29, 48, 57, 81 | 14, 31, 48, 71, 78 | ||||
| 6, 22, 38, 54, 87 | 16, 32, 48, 64, 80 | 10, 27, 44, 61, 78 | 8, 23, 38, 68, 79 | 2, 17, 48, 63, 78 | 8, 25, 40, 57, 72 | 2, 20, 38, 56, 74 | 12, 30, 50, 68, 88 | 14, 28, 42, 71, 86 | 8, 22, 36, 80, 84 | ||||
| 5, 21, 37, 69, 85 | 15, 31, 47, 63, 79 | 9, 26, 43, 60, 77 | 7, 22, 37, 67, 87 | 1, 32, 38, 62, 77 | 7, 24, 39, 56, 80 | 1, 19, 37, 55, 63 | 11, 29, 47, 67, 76 | 13, 27, 41, 55, 69 | 7, 21, 35, 79, 83 | ||||
| 4, 20, 36, 68, 84 | 30, 50, 58, 62, 78 | 8, 25, 42, 59, 76 | 6, 21, 36, 66, 85 | 16, 31, 46, 61, 76 | 6, 23, 38, 55, 70 | 16, 17, 48, 80, 81 | 10, 28, 46, 64, 78 | 12, 50, 40, 54, 80 | 6, 20, 34, 64, 78 | ||||
| 3, 19, 35, 67, 78 | 13, 29, 45, 61, 77 | 7, 24, 41, 75, 83 | 5, 20, 35, 61, 65 | 15, 30, 45, 60, 75 | 5, 22, 37, 54, 76 | 15, 32, 47, 64, 79 | 9, 27, 45, 57, 63 | 11, 25, 39, 67, 85 | 5, 19, 33, 51, 77 | ||||
| 2, 18, 34, 61, 66 | 12, 28, 44, 60, 76 | 6, 23, 40, 57, 74 | 16, 17, 34, 68, 84 | 14, 29, 44, 74, 82 | 4, 21, 36, 68, 85 | 10, 31, 46, 54, 78 | 8, 26, 62, 73, 80 | 10, 24, 38, 66, 87 | 4, 18, 48, 62, 76 | ||||
| 1, 17, 33, 49, 65 | 11, 23, 27, 59, 75 | 5, 22, 39, 56, 85 | 15, 32, 33, 50, 67 | 13, 28, 43, 55, 81 | 3, 20, 35, 67, 78 | 13, 30, 45, 62, 77 | 7, 25, 43, 61, 79 | 9, 23, 37, 61, 65 | 3, 17, 47, 61, 75 | ||||
| ѕред- | послед-н€€ | цифра |
“ е м а: ’имическа€ термодинамика
|
|
|
ѕримеры решени€ задач
«адача 1. –ассчитайте количество теплоты, выделившейс€ при аэробном окислении 18 г глюкозы, если ее стандартна€ энтальпи€ сгорани€ равна Ц2805 кƒж/моль.
–ешение
ѕроцесс аэробного окислени€ глюкозы можно представить как процесс ее сгорани€ при посто€нном давлении (р = 101,325 кѕа)
—6Ќ12ќ6(т) + 6 ќ2(г) = 6 —ќ2(г) + 6 Ќ2ќ(ж), 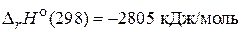 .
.
ѕри сгорании 1 моль глюкозы, по условию задачи, выдел€етс€ 2805 кƒж/моль теплоты. ћол€рна€ масса глюкозы 180 г/моль, поэтому 18 г глюкозы составл€ют 0,1 моль. –ассчитаем количество теплоты, выделившеес€ при сгорании 18 г глюкозы:
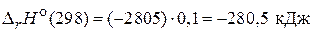 .
.
«адача 2. ¬ растени€х происходит гидролитическое расщепление мочевины на аммиак и оксид углерода (IV). —равните 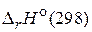 и
и 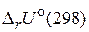 дл€ реакции гидролиза мочевины (стандартные энтальпии образовани€ (
дл€ реакции гидролиза мочевины (стандартные энтальпии образовани€ (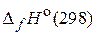 ) участников реакции приведены в таблице 1):
) участников реакции приведены в таблице 1):
“ а б л и ц а 1
| ¬ещество | 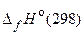 , кƒж/моль , кƒж/моль
|
| CO(NH2)2 (т) | Ц333,20 |
| Ќ2ќ (ж) | Ц285,85 |
| —ќ2(г) | Ц393,50 |
| NЌ3(г) | Ц47,70 |
акой физический смысл имеют величины 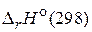 и
и 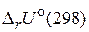 дл€ химической реакции?
дл€ химической реакции?
–ешение
«апишем уравнение реакции гидролиза мочевины:
CO(NH2)2(т) + Ќ2ќ(ж) = —ќ2(г) + 2 NЌ3(г) .
ƒл€ расчета используем 1-е следствие из закона √есса, согласно которому изменение энтальпии реакции равно разности сумм изменени€ энтальпий образовани€ продуктов реакции и исходных веществ с учетом стехиометрических коэффициентов.
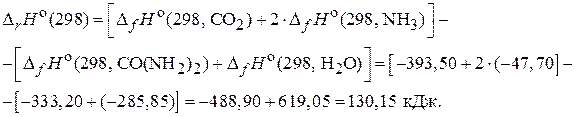
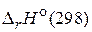 и
и 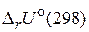 св€заны между собой соотношением:
св€заны между собой соотношением:
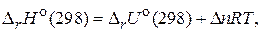
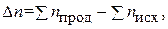
где n Ц число молей газообразных участников реакции.
|
|
|
ƒл€ данной реакции 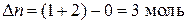 .
.
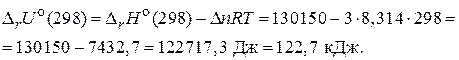
¬еличины 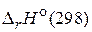 и
и 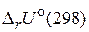 дл€ химической реакции характеризуют тепловой эффект при P = const и V = const, соответственно. Cравнива€ их можно заметить, что тепловой эффект реакции при посто€нном давлении больше, чем при посто€нном объеме. ѕоскольку
дл€ химической реакции характеризуют тепловой эффект при P = const и V = const, соответственно. Cравнива€ их можно заметить, что тепловой эффект реакции при посто€нном давлении больше, чем при посто€нном объеме. ѕоскольку 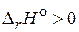 , реакци€ €вл€етс€ эндотермической, т. е. идет с поглощением теплоты.
, реакци€ €вл€етс€ эндотермической, т. е. идет с поглощением теплоты.
«адача 3. –ассчитайте энтальпию образовани€ ацетона, если при сгорании 1 моль его выдел€етс€ 1785,73 кƒж/моль теплоты, а стандартные энтальпии образовани€ Ќ2ќ(ж) и —ќ2(г) соответственно равны Ц285,85 кƒж/моль и Ц393,50 кƒж/моль.
–ешение
Ёнтальпи€ образовани€ ацетона представл€ет собой тепловой эффект реакции образовани€ 1 моль ацетона из простых веществ:
3—(т) + 3Ќ2(г) + 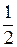 ќ2(г) Ѓ —Ќ3—ќ—Ќ3(ж) (1)
ќ2(г) Ѓ —Ќ3—ќ—Ќ3(ж) (1)
—оставим термохимические уравнени€ дл€ приведенных значений энтальпий:
—Ќ3—ќ—Ќ3(ж) + 4ќ2(г) = 3—ќ2(г) + 3Ќ2ќ(ж); 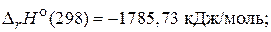 (2)
(2)
—(т) + ќ2(г) = —ќ2(г); 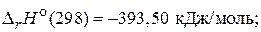 (3)
(3)
Ќ2(г) + 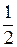 ќ2(г) = Ќ2ќ(ж);
ќ2(г) = Ќ2ќ(ж); 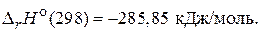 (4)
(4)
»з уравнений (3) и (4) можно заключить, что энтальпи€ образовани€ —ќ2 и Ќ2ќ €вл€етс€ энтальпией сгорани€ углерода и водорода.
Ќа основании приведенных уравнений (2) - (4) можно сказать, что в задаче известны энтальпии сгорани€ всех участников реакции (1). ѕоэтому дл€ расчета энтальпии образовани€ ацетона применим 2-е следствие из закона √есса, согласно которому изменение энтальпии реакции равно разности сумм энтальпий сгорани€ исходных веществ и продуктов реакции с учетом стехиометрических коэффициентов:
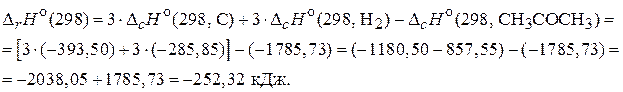
«начение 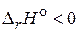 , следовательно, реакци€ экзотермическа€.
, следовательно, реакци€ экзотермическа€.
«адача 4. –ассчитайте 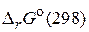 дл€ реакции образовани€ дипептида из глицина и определите направление самопроизвольного протекани€ процесса. ƒл€ расчета используйте данные, приведенные в таблице 2.
дл€ реакции образовани€ дипептида из глицина и определите направление самопроизвольного протекани€ процесса. ƒл€ расчета используйте данные, приведенные в таблице 2.
“ а б л и ц а 2
| ¬ещество | 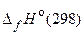 , кƒж/моль , кƒж/моль
| 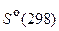 , ƒж/(моль× ) , ƒж/(моль× )
|
| глицин | Ц537,23 | 103,00 |
| глицилглицин | Ц746,01 | 189,95 |
| Ќ2ќ(ж) | Ц285,85 | 69,54 |
–ешение
«апишем уравнение реакции:
Ќ2NЧ CH2ЧCOOH + Ќ2NЧ CH2ЧCOOH =
= Ќ2NЧ CH2ЧCOЧЌNЧ CH2ЧCOOH + H2O.
–ассчитаем изменение энтальпии по 1-му следствию из закона √есса:
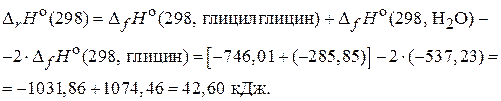
»зменение энтропии рассчитаем по формуле:
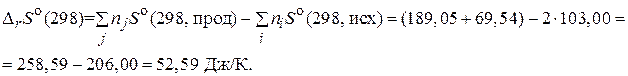 ѕо уравнению √иббса - √ельмгольца находим изменение
ѕо уравнению √иббса - √ельмгольца находим изменение 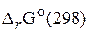 :
:
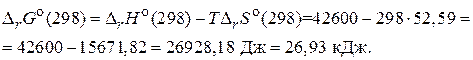
Ќа основании значени€ 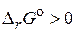 можно заключить, что данна€ реакци€ самопроизвольно протекать не может, т. е. предпочтительнее ее протекание в обратном направлении.
можно заключить, что данна€ реакци€ самопроизвольно протекать не может, т. е. предпочтительнее ее протекание в обратном направлении.
¬опросы и задани€
1. —формулируйте первый закон термодинамики. ƒайте определение по≠н€тий "теплота", "работа", "внутренн€€ энерги€ системы", "энтальпи€".
2. „то называетс€ тепловым эффектом химической реакции и какие методы его определени€ вы знаете? ѕриведите примеры реакций, дл€ которых: а) 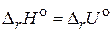 ; б)
; б) 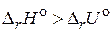 ; в)
; в) 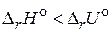 .
.
3. ћожно ли рассматривать закон √есса как следствие из первого закона термодинамики в приложении его к химическим процессам?
4. „то понимают под стандартной энтальпией образовани€ и стандартной энтальпией сгорани€ веществ? Ќапишите уравнени€ реакций, по€сн€ющих физический смысл стандартных энтальпий образовани€ следующих веществ: H2O, CO2, C6H12O6.
5. ”кажите какими трем€ основными пут€ми расходуетс€ энерги€, поглощаема€ человеком в виде пищи.
6. „то понимают под стандартной энтальпией сгорани€? ћожно ли использовать эту величину дл€ оценки энергетики окислительных процессов?
7. ѕриведите формулировки и математическую запись следствий из закона √есса. ¬ чем их практическое значение?
8. „то понимают под внутренней энергией и энтальпией системы? ѕокажите св€зь между 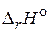 и
и 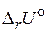 .
.
9. ¬ каких услови€х протекают следующие процессы: а) сгорание метана в закрытом стальном сосуде, который погружен в емкость с водой; б) окисление глюкозы в нашем организме; в) сгорание глюкозы на воздухе? какому типу можно отнести каждую из этих систем?
10. Hапишите уравнение, выражающее св€зь между тепловым эффектом при посто€нном давлении и тепловым эффектом при посто€нном объеме.
11. ћожно ли экспериментально определить и теоретически рассчитать абсолютные значени€ внутренней энергии и энтальпии системы?
|
|
|
12. акие процессы называют экзергоническими и эндергоническими?
13. «ависит ли тепловой эффект химической реакции от числа стадий при изобарно-изотермических услови€х проведени€ процесса?
14. ¬ организме человека окисление этилового спирта протекает в две стадии:
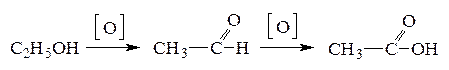
¬ ходе первой стадии выдел€етс€ 256 кƒж/моль, а в ходе второй Ч 237 кƒж/моль теплоты. –ассчитайте тепловой эффект процесса окислени€ этанола до уксусной кислоты. акие выводы можно сделать о вреде потреблени€ алкогол€, если учесть, что окисление уксусного альдегида в уксусную кислоту протекает во времени, а присутствие уксусного альдегида в крови вызывает затруднение дыхани€, сбои пульса, тошноту, чувство жара в голове и груди? (Ц493 кƒж/моль)
15. ћожно ли экспериментально определить изменение внутренней энергии при сгорании глюкозы?
16. акие системы называют открытыми, закрытыми, изолиро-ванными? каким системам можно отнести живые организмы?
17. ƒл€ каких расчетов примен€ют "стандартную энергию √иббса образовани€ веществ" (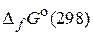 )?
)?
18. ѕочему термодинамика рассматривает не абсолютные значени€ внутренней энергии, а только ее изменение?
19. ѕочему дл€ конденсированных систем разница между энтальпией и внутренней энергии мала, а дл€ газообразных систем значительна?
20. акие термодинамические функции примен€ют дл€ определени€ направлени€ самопроизвольного протекани€ процессов в открытых и закрытых системах?
21. ƒл€ каких расчетов используют термодинамические потенциалы?
22. акие термодинамические функции €вл€ютс€ критери€ми дл€ определени€ направлени€ самопроизвольного протекани€ процесса в услови€х а) –,“ = const, б) V,T = const?
23. ѕредскажите изменение энтропии в следующих процессах: а) плавление льда; б) кипение воды; в) Ќ2(г) + 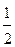 ќ2(г) = Ќ2ќ(г).
ќ2(г) = Ќ2ќ(г).
24. ѕриведите формулировки и математическое выражение второго закона термодинамики.
25. ѕриведите примеры самопроизвольных и несамопроизвольных процессов.
26. ћогут ли в организме осуществл€тьс€ эндергонические процессы? ќтвет обоснуйте.
27. ак св€зана константа равновеси€ реакции с изменением функции √иббса?
28. ѕриведите несколько формулировок второго закона термодинамики, напишите его математическое выражение. ¬ чем состоит значение второго закона термодинамики дл€ процессов, протекающих в живых организмах?
29. ћожно ли, использу€ термодинамические параметры реакции, провести оценку выхода продуктов (рассчитать константу равновеси€)?
30. акие из приведенных реакций могут протекать в пр€мом, а какие - в обратном направлении при “, – = сonst:
а) SO2(г) + 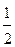 O2(г)= SO3(г),
O2(г)= SO3(г), 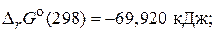
б) 2H—l(г) = H2(г) + Cl2(г), 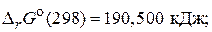
в) 2H2(г) + O2(г) = 2H2O(г), 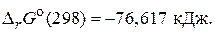
31. »зменение энтальпии дл€ реакции H2(г) + 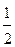 O2(г) = H2O(ж) равно
O2(г) = H2O(ж) равно
Ц285,85 кƒж/моль при 298 и 101,325 кѕа. ѕредскажите вли€ние температуры на константу равновеси€ этой реакции.
32. Ќазовите критерии самопроизвольности протекани€ процессов и равновеси€ в изолированных и неизолированных системах.
33. —тандартные энтальпии образовани€ газообразного оксида углерода(IV) и жидкой воды равны соответственно Ц393,0 и Ц285,85 кƒж/моль. акова энергетическа€ ценность (кƒж/г) углерода и водорода как видов топлива? (32,75 кƒж/г; 142,9 кƒж/г)
34. — ростом температуры равновесие реакции SO2+ 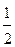 O2=SO3 смещаетс€ влево. —делайте вывод о знаке теплового эффекта реакции. ќтвет обоснуйте.
O2=SO3 смещаетс€ влево. —делайте вывод о знаке теплового эффекта реакции. ќтвет обоснуйте.
35. ¬ли€ет ли давление на выход продуктов в обратимой реакции?
36. ак отличаютс€ изобарный и изохорный тепловые эффекты дл€ реакции образовани€ жидкой воды?
37. ак вли€ет температура на величину константы равновеси€ ( р) экзотермической реакции?
38. Ёнергетическа€ ценность сахара составл€ет 16,80 кƒж/г. ќбъ€сните возможные пути экспериментального определени€ или теоретического расчета этой величины?
|
|
|
39. аким образом можно увеличить выход оксида азота (IV) в реакци€х, идущих по уравнени€м:
1) 2Nќ(г) + ќ2(г) = 2Nќ2(г) , 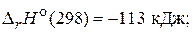
2) N2ќ4(г) = 2Nќ2(г), 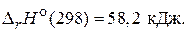
40. ќбъ€сните изменение выхода продуктов с повышением температуры дл€ газообразных реакций, протекающих: а) с выделением теплоты, 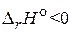 ; б) с поглощением теплоты,
; б) с поглощением теплоты, 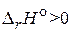 . ƒл€ объ€снени€ используйте уравнение изобары ¬антЦ√оффа.
. ƒл€ объ€снени€ используйте уравнение изобары ¬антЦ√оффа.
41. „ем объ€снить посто€нство теплоты нейтрализации сильной кислоты сильным основанием?
42. ќдинаковые ли будут тепловые эффекты при нейтрализации серной кислоты растворами: а) гидроксида натри€; б) гидроксида кали€; в) аммиака?
43. акова св€зь между изменени€ми внутренней энергии и энтальпией системы? ѕо€сните на конкретных примерах.
44. ѕримен€€ математическое выражение первого закона термодинамики, покажите, что тепловой эффект химической реакции при посто€нном давлении есть изменение энтальпии, а тепловой эффект химической реакции при посто€нном объеме - изменение внутренней энергии химической реакции.
45. „то означает пон€тие "стационарное" состо€ние организма и чем оно отличаетс€ от равновесного состо€ни€ системы?
46. ¬ каком направлении сместитс€ равновесие при изменении давлени€ и температуры в реакци€х:
1) 2—O(г) + O2(г) = 2—O2(г), 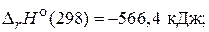
2) —O2(г) + —(т) = 2—O(г), 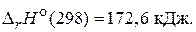
47. ѕрименимы ли основные законы термодинамики к живым организмам?
48. Hе противоречит ли уравнение изобары химической реакции принципу Ће Ўателье?
49. —огласно первому закону термодинамики теплота есть функци€ процесса. «акон √есса утверждает, что теплота химической реакции не зависит от пути процесса. ќбъ€сните это УпротиворечиеФ.
50. ”кажите факторы, смещающие равновесие и не измен€ющие константу равновеси€.
51. акие процессы характеризуютс€ изменением функции √ельмгольца?
52. лассифицируйте следующие реакции по знаку энтальпии:
C2H4(г) + H2O(г) = C2H5OH(г), 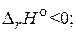
CO2(г) + 2H2S(г) = 2H2O(г) + CS2(г), 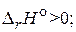
3H2(г) + CO(г) = CH4(г) + H2O(г), 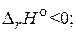
2H2O(г) + 3CO(г) = CH3OH(г) + 2CO2(г), 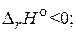
CaCO3(т) = CaO(т) + CO2(г), 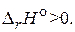
53. »з приведенных процессов укажите те, дл€ которых 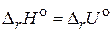 :
:
4HCl(г) + O2(г) = 2H2O(г) + 2Cl2(г);
2NO2(г) + O3(г) = O2(г) + N2O5(г);
CH4(г) + 2O2(г) = CO2(г) + 2H2O(г);
2H2(г) + 2CO(г) = CH4(г) + CO2(г);
H2(г) + Cl2(г) = 2HCl(г).
54. ака€ из приведенных ниже реакций поставл€ет организму больше энергии:
1) C6H12O6(т) = 2C2H5OH(ж) + 2CO2(г);
2) C6H12O6(т) + 6O2(г) = 6CO2(г) + 6H2O(ж).
55. ќпределите калорийность пищевого продукта массой 380 г, содержащего 15 % углеводов, 20 % жиров, 35 % белков и 30 % воды, если калорийность белков равна 17,1 кƒж/г, жиров Ц 38,0 кƒж/г, углеводов 17,1 кƒж/г. (6137 кƒж)
56. ¬ычислите стандартную энтальпию образовани€ сахарозы —12Ќ22ќ11, если тепловые эффекты реакций сгорани€ веществ соответственно равны:
C12H22O11(т) + 12O2(г)= 12—ќ2(г) + 11H2O(ж), 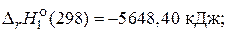
—(т) + O2(г) = CO2(г), 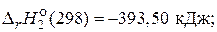
Ќ2(г)+ 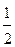 O2(г) = H2O(ж),
O2(г) = H2O(ж), 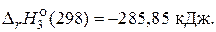
(Ц2218 кƒж/моль)
57. ѕри сгорании некоторого количества глюкозы на открытом воздухе выделилось 563,17 кƒж теплоты. Ќайдите количество прореагировавшей глюкозы, если известно, что энтальпи€ ее сгорани€ Ц2815,83 кƒж/моль. (0,2 моль)
58. Ќе провод€ расчетов, покажите отличие изобарного и изохорного тепловых эффектов следующих реакций:
1) —Ќ4(г) + 2ќ2(г) = —ќ2(г) + 2Ќ2ќ(ж);
2) N2(г) + 3Ќ2(г) = 2NH3(г).
59. –ассчитайте количество теплоты, выдел€ющейс€ при гашении 1 кг извести водой, если стандартные энтальпии образовани€ (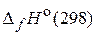 ) —аќ(т), —а(ќЌ)2(т) и Ќ2ќ(ж) соответственно равны Ц635,10; Ц986,20; Ц285,85 кƒж/моль. (1165,18 кƒж)
) —аќ(т), —а(ќЌ)2(т) и Ќ2ќ(ж) соответственно равны Ц635,10; Ц986,20; Ц285,85 кƒж/моль. (1165,18 кƒж)
60. акое количество теплоты выделитс€ при сгорании 150 г этилового спирта, если стандартные энтальпии образовани€ —2Ќ5ќЌ(ж), —ќ2(г) и Ќ2ќ(ж) равны соответственно Ц277,80; Ц393,50 и Ц285,85 кƒж/моль. (4456,8 кƒж)
61. Ќайдите разность между qр и qv при 25 ∞— дл€ следующих реакций:
1) CO(г) + 3H2(г) = CH4(г) + H2O(ж);
2) C2H5OH(ж) = C2H4(г) + H2O(ж).
62. ѕри сгорании 1 л ацетилена при посто€нном объеме и 298 выдел€етс€ 58,0 кƒж теплоты. ћожно ли на основании этих данных вычислить стандартную энтальпию сгорани€ ацетилена?
63. ѕри горении в стандартных услови€х 2 г водорода выдел€етс€ 285,85 кƒж теплоты. –ассчитайте количество выдел€емой теплоты при проведении реакции в закрытом сосуде. (282,3 кƒж)
64. ѕри полном сгорании 46 г этилового спирта выдел€етс€ 1367,7 кƒж теплоты. –ассчитайте стандартную энтальпию образовани€ этилового спирта, если стандартные энтальпии образовани€ воды жидкой и углекислого газа равны соответственно Ц285,85 и Ц393,50 кƒж/моль. (Ц276, 85 кƒж/моль)
65. –ассчитайте стандартную энтальпию образовани€ сахарозы —12H22ќ11 (тв), если тепловой эффект реакции
|
|
|
—12H22ќ11 (тв) + 12ќ2 (тв) = 12—ќ2 (г) + 11H2ќ(ж)
равен Ц5694 кƒж, и стандартные энтальпии образовани€ H2ќ(ж) и —ќ2(г) соответственно равны Ц285,85 и Ц393,50 кƒж/моль. (Ц2172, 35 кƒж/моль)
66. „ему равно изменение энтальпии реакции сгорани€ водорода при посто€нном объеме, если ее тепловой эффект при посто€нном давлении и при 25 ∞— равен 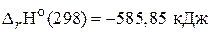 ? (Ц282,3 кƒж)
? (Ц282,3 кƒж)
67. –ассчитайте энтальпию образовани€ пальмитиновой кислоты (C15H31COOH), если при сгорании 1 моль ее при 298 выдел€етс€ 9796 кƒж теплоты, а стандартные энтальпии образовани€ CO2(г) и H2ќ(ж) соответственно равны Ц393,5 и Ц285,85 кƒж/моль.(Ц1073,6 кƒж/моль)
68. —тандартна€ энтальпи€ сгорани€ метана равна Ц822 кƒж/моль. –ассчитайте количество теплоты, выделившейс€ при сгорании 160 г метана. (8220 кƒж)
69. –еакци€ горени€ ацетилена при стандартных услови€х сопровождаетс€ выделением 1300 кƒж теплоты. –ассчитайте стандартную энтальпию образовани€ ацетилена при посто€нном давлении, если:
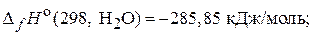
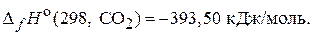
(Ц228,15 кƒж/моль)
70. ¬ычислите изменение энтальпии реакции при 25 ∞—
—2H5ќH(ж) + O2(г) = CH3COOH(ж) + H2O(ж),
если стандартные энтальпии сгорани€ этилового спирта и уксусной кислоты равны соответственно Ц1367,7 и Ц872,1 кƒж/моль. (Ц495,6 кƒж)
71. Ѕудут ли отличатьс€ изобарный и изохорный тепловые эффекты реакций:
1) N2(г) + O2(г) = 2NO(г); 2) —6Ќ12ќ6(т) + 6ќ2(г) = 6CO2(г) + 6H2O(ж).
72. —тандартное изменение функции √иббса реакции горени€ глюкозы равна Ц2880 кƒж. ѕри дыхании часть этой энергии в количестве 1140 кƒж запасаетс€ клетками дл€ выполнени€ работы. —колько энергии запасут клетки при разложении 0,5 г глюкозы? (3,16 кƒж)
73. ¬озможен ли самопроизвольный процесс образовани€ глюкозы из углекислого газа и воды при посто€нных температуре и давлении? ќтвет обоснуйте.
74. –ассчитайте константы равновеси€ р и с реакции N2O4(г) = 2NO2(г), если стандартные изобарные потенциалы образовани€ (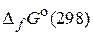 ) дл€ NO2(г) и N2O4(г) равны 51,84 и 98,82 кƒж/моль соответственно. (0,14; 5,65Ј10Ц5)
) дл€ NO2(г) и N2O4(г) равны 51,84 и 98,82 кƒж/моль соответственно. (0,14; 5,65Ј10Ц5)
75. –ассчитайте изменение энтропии процесса обмена карбоксиметилцеллюлозы с ионами +(Na+) при 300 , если при этом выдел€етс€ 23 кƒж теплоты, а прирост функции √иббса составил 0,66 кƒж. акие выводы можно сделать о направлении св€зывани€ карбоксиметилцеллюлозы с ионами +(Na+)? (Ц78,87 ƒж/ )
76. онстанты равновеси€ ( р) двух реакций равны соответственно 1 и 5×10Ц3. –ассчитайте изменение изобарноЦизотермического потенциала и сделайте вывод о направлении самопроизвольного протекани€ каждой реакции. (0 кƒж/моль; 13,127 кƒж/моль)
77. –ассчитайте изменение энтропии при реакции гидролиза ј“‘ в живом организме ј“‘ + H2O = јƒ‘ + H–O42Ц + H+, если при этих услови€х выделилось 20 кƒж теплоты, а убыль изобарноЦизотермического потенциала составила 30 кƒж. (Ц33,56 ƒж/ )
78. Ёнергетическа€ ценность белков составл€ет 17 кƒж/г, а жиров - 38 кƒж/г. Ћюд€м, зан€тым т€желым физическим трудом, необходимо поглощать с пищей ~ 10500 кƒж ежедневно. –ассчитайте массу жиров в суточном рационе человека, если белков в нем содержалось 300 г. (142 г)
79. онстанта равновеси€ реакции аланина с глицином, привод€ща€ к образованию дипептидной св€зи, равна 1Ј10Ц3. ћожет ли данный процесс идти самопроизвольно при 298 ?
80. ѕри растворении аденина в воде и метаноле при 298 поглощаетс€ 29,58 и 16,57 кƒж/моль теплоты, а прирост энтропии при растворении в воде и метаноле составл€ет 25,5 и 39,3 ƒж/(моль× ) соответственно. ¬ каком из этих растворителей лучше раствор€етс€ аденин?
81. –ассчитайте тепловой эффект и изменение энтропии реакции разложени€ карбоната кальци€ на открытом воздухе, использу€ данные таблицы 3.
“ а б л и ц а 3
| ¬ещество | 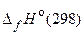 , кƒж/моль , кƒж/моль
| 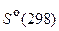 , ƒж/(моль× ) , ƒж/(моль× )
|
| —ќ2(г) | Ц393,51 | 213,82 |
| —аќ(г) | Ц635,09 | 39,77 |
| —а—ќ3(г) | Ц1206,83 | 88,8 |
ћожет ли протекать самопроизвольно эта реакци€ при температуре 25 ∞—? (178,23 кƒж; 164,79 ƒж/ )
82. –ассчитайте энтальпию образовани€ сероуглерода, использу€ термохимические уравнени€ следующих реакций:
S(т) + O2(г) = SO2(г), 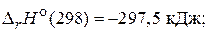
CS2(т) + 3O2(г) = CO2(г)+ 2SO2(г), 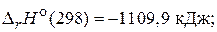
—(т) + O2(г) = CO2(г), 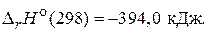
(Ц120,9 кƒж/моль)
83. —тандартное изменение энтальпии сгорани€ кристаллической аминоуксусной кислоты при 298 равно Ц976,7 кƒж/моль. ѕроцесс сгорани€ протекает по схеме
NH2CH2COOH(т) + 2 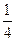 ќ2 = 2—ќ2(г) + 2
ќ2 = 2—ќ2(г) + 2 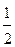 Ќ2ќ (ж)+
Ќ2ќ (ж)+ 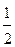 N2(г)
N2(г)
–ассчитайте изменение стандартной энтальпии образовани€ аминоуксусной кислоты при заданной температуре, если
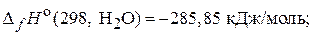
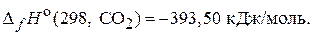
(Ц524,9 кƒж/моль)
84. Ёнергетическа€ ценность сливочного масла 30,9 кƒж/г, а белого хлеба 9,7 кƒж/г. —колько энергии получит ¬аш организм при потреблении бутерброда, состо€щего из 100 г хлеба и 20 г масла? (1588 кƒж)
85. ќцените возможность образовани€ газообразного метанола из оксида углерода (II) и водорода при 298 , использу€ данные таблицы 4:
“ а б л и ц а 4
| ¬ещество | 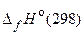 , кƒж/моль , кƒж/моль
| 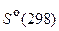 , ƒж/(моль× ) , ƒж/(моль× )
|
| —ќ(г) | Ц110,60 | 197,68 |
| Ќ2(г) | 130,66 | |
| —Ќ3ќЌ(г) | Ц202,10 | 239,90 |
86. ѕри изучении равновеси€ реакции —(т) + 2H2(г) = CH4(г) значени€ констант равновеси€ при температурах 700 и 750 ∞— равны 0,195 и 0,118. –ассчитайте тепловой эффект этой реакции и сравните с наиболее точным значением (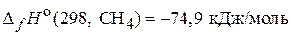 ). (Ц83,14 кƒж)
). (Ц83,14 кƒж)
87. ƒайте термодинамическую оценку возможности получени€ анилина из хлорбензола и аммиака —6H5Cl(ж) + NH3(г) = —6H5NH2(ж) + HCl(г) в стандартных услови€х, если стандартные изменени€ функции √иббса образовани€ —6H5Cl(ж), NH3(г), —6H5NH2(ж), HCl(г) соответственно равны: 198,4; Ц16,63; 153,1; Ц95,28 кƒж/моль.
88. ¬ычислите изменение энтропии реакции при стандартных услови€х:
4HCl(г) + O2(г) = 2H2O(г) + 2Cl2(г),
если абсолютные значени€ энтропии HCl(г), O2(г), H2O(г), Cl2(г) равны соответственно 186,92; 205,17; 188,85; 223,13 ƒж/(мольЈ ). (Ц128,89 ƒж/ )
| ¬арианты индивидуальных заданий дл€ контрольной работы по теме: У’имическа€ кинетикаФ | Ќомера вопросов | ѕоследн€€ цифра | 15, 24, 32, 61, 81 | 5, 8, 9, 63, 76 | 33, 35, 37, 59, 65 | 18, 23, 50, 55, 82 | 44, 46, 47, 71, 84 | 2, 11, 42, 57, 86 | 22, 39, 45, 74, 79 | 7, 10, 21, 36, 53 | 22, 41, 49, 60, 64 | 2, 20, 32, 67, 83 | |
| 22, 28, 50, 58, 79 | 4, 7, 47, 60, 75 | 20, 26, 30, 64, 88 | 10, 13, 49, 54, 80 | 36, 39, 45, 68, 81 | 1, 9, 32, 51, 85 | 19, 38, 41, 72, 77 | 2, 6, 33, 75, 87 | 12, 19, 40, 59, 62 | 28, 31, 48, 65, 87 | ||||
| 19, 25, 49, 57, 77 | 3, 45, 48, 59, 74 | 17, 29, 43, 62, 84 | 6, 12, 14, 53, 78 | 2, 38, 41, 67, 79 | 8, 28, 43, 56, 83 | 18, 35, 40, 71, 73 | 5, 34, 44, 72, 86 | 11, 18, 37, 61, 88 | 8, 25, 46, 64, 76 | ||||
| 12, 18, 23, 56, 73 | 41, 44, 46, 72, 86 | 24, 27, 42, 61, 81 | 5, 11, 31, 52, 76 | 1, 35, 40, 65, 77 | 25, 43, 46, 54, 82 | 14, 26, 37, 68, 70 | 1, 4, 20, 68, 85 | 9, 14, 30, 58, 84 | 23, 39, 50, 62, 72 | ||||
| 10, 11, 14, 55, 70 | 36, 39, 40, 71, 85 | 16, 22, 32, 58, 79 | 4, 8, 9, 51, 75 | 20, 26, 37, 64, 73 | 21, 23, 24, 39, 80 | 13, 17, 30, 67, 69 | 3, 44, 50, 71, 82 | 12, 29, 34, 57, 81 | 10, 38, 49, 61, 86 | ||||
| 6, 9, 13, 54, 69 | 2, 37, 38, 68, 82 | 15, 19, 28, 57, 77 | 3, 7, 21, 44, 74 | 17, 30, 34, 62, 70 | 10, 22, 38, 55, 78 | 29, 31, 33, 59, 65 | 31, 36, 49, 67, 83 | 27, 42, 50, 56, 79 | 6, 12, 35, 58, 66 | ||||
| 5, 31, 44, 53, 66 | 1, 30, 35, 67, 83 | 18, 25, 50, 56, 73 | 36, 47, 48, 72, 87 | 29,42, 43, 61, 69 | 6, 19, 35, 52, 76 | 8, 27, 34, 63, 64 | 2, 8, 12, 65, 80 | 16, 32, 49, 55, 77 | 5, 11, 26, 57, 85 | ||||
| 4, 8, 36, 52, 63 | 26, 29, 34, 65, 80 | 14, 23, 49, 55, 70 | 2, 45, 46, 71, 86 | 24, 27, 32, 58, 66 | 5, 18, 26, 53, 75 | 16, 20, 50, 60, 62 | 1, 7, 11, 78, 88 | 12, 15, 28, 54, 73 | 4, 9, 17, 56, 74 | ||||
| 2, 3, 7, 51, 60 | 17, 20, 27, 64, 87 | 10, 12, 13, 54, 69 | 1, 39, 41, 68, 85 | 16, 22, 28, 57, 63 | 4, 14, 17, 59, 74 | 15, 44, 49, 61, 66 | 9, 43, 48, 76, 84 | 11, 25, 31, 52, 69 | 3, 43, 47, 53, 71 | ||||
| 1, 17, 21, 33, 59 | 16, 42, 43, 62, 78 | 6, 11, 31, 53, 66 | 34, 38, 40, 67, 83 | 15, 19, 25, 56, 60 | 3, 13, 48, 72, 88 | 7, 12, 36, 58, 87 | 24, 46, 47, 75, 81 | 8, 9, 23, 51, 74 | 24, 45, 50 52, 63, | ||||
| ѕред- | послед-н€€ | цифра |
“ е м а: ’имическа€ кинетика
ѕримеры решени€ задач
«адача 1. “емпературный коэффициент скорости реакции гидролиза некоторого лекарственного препарата равен 2,5. ѕри какой температуре нужно провести его Уискусственное старениеФ, чтобы скорость разрушени€ препарата возросла в 10 раз? (–екомендуема€ температура хранени€ 20 ∞—).
–ешение
—огласно правилу ¬антЦ√оффа при увеличении температуры на каждые 10 ∞ скорость реакции возрастает в 2 - 4 раза:
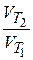 =
= 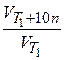 = g n,
= g n,
где n = 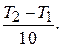
“ак как отношение скоростей при “ 2 и “ 1 = 293 равно 10, а g = 2,5,
то рассчитаем величину n:
g n = 10; 2,5 n = 10.
ѕрологарифмируем данное выражение:
n lg 2,5 = lg 10,
n = 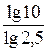 =
= 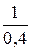 = 2,5.
= 2,5.
T 2 = 10 n + T 1 = 10×2,5 + 293 = 318,1 K (45,1∞C).
«адача 2. ’лористый фенилдиазоний при 50 ∞C в воде подвергаетс€ термическому распаду по уравнению первого пор€дка с константой скорости равной 0,07 минЦ1. —колько времени нужно нагревать раствор при указанной температуре, чтобы исходна€ концентраци€ вещества, равна€ 0,01 моль/л, уменьшилась в 10 раз? „ему равен период полураспада фенилдиазони€ при 50 ∞C?
–ешение
«апишем выражение дл€ константы скорости реакции первого пор€дка:
k = 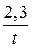 lg
lg 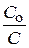 .
.
ќтсюда t = 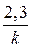 lg
lg 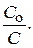
“екуща€ концентраци€ будет равна: — = 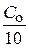 = 0,1×0,01 = 0,001 моль/л
= 0,1×0,01 = 0,001 моль/л
t = 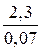 lg
lg 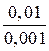 = 32,86 мин.
= 32,86 мин.
ѕериод полураспада, т. е. врем€, за которое начальна€ концентраци€ уменьшаетс€ в 2 раза, дл€ реакции первого пор€дка рассчитываем по формуле
t1/2 = 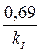 =
= 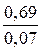 = 9,86 мин.
= 9,86 мин.
«адача 3. –ассчитайте энергию активации реакции, дл€ которой при повышении температуры с 295 до 305 скорость реакции удваиваетс€.
–ешение
»спользуем уравнение јррениуса в интегральной форме:
lg 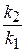 =
= 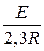 (
(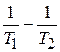 );
);
≈сли скорость реакции удваиваетс€, то 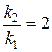 ;
;
E = 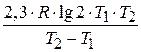 =
= 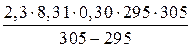 = 51,59 кƒж/моль.
= 51,59 кƒж/моль.
¬опросы и задани€
1. ƒайте определение пон€ти€ скорости химической реакции и перечислите факторы, вли€ющие на ее величину.
2. —формулируйте закон действующих масс дл€ скорости химической реакции. аков физический смысл константы скорости реакции?
3. „то понимают под молекул€рностью и пор€дком химической реакции? ¬сегда ли они совпадают? ѕриведите примеры.
4. акие реакции относ€тс€ к реакци€м первого пор€дка? ѕриведите уравнение дл€ расчета константы скорости этих реакций.
5. акие реакции называютс€ реакци€ми нулевого пор€дка? ѕриведите уравнение дл€ расчета константы скорости.
6. акие реакции относ€тс€ к реакци€м второго пор€дка? ѕриведите уравнени€ дл€ расчета константы скорости этих реакций.
7. „то называетс€ периодом полураспада? ѕокажите характер зависимости периода полураспада от начальной концентрации в реакци€х нулевого, первого и второго пор€дков.
8. акие экспериментальные данные необходимо иметь дл€ определени€ константы скорости?
9. ак вли€ет температура на скорость химической реакции? »зобразите графически известные вам зависимости скорости от температуры.
10. Ќапишите кинетическое уравнение реакции:
FeCl3 + 3 KNCS = 3 K—l + Fe(NCS)3,
если известно, что она имеет первый пор€док по концентраци€м обоих реагирующих веществ.
11. акими уравнени€ми описываетс€ вли€ние температуры на скорость реакции?
12. ¬ чем заключаютс€ особенности вли€ни€ температуры на скорость реакций, протекающих в живых организмах (биологических системах)?
13. ѕриведите график изменени€ энергии системы в ходе химической реакции.
14. акие экспериментальные данные необходимо иметь дл€ определени€ энергии активации?
15. ѕриведите примеры простых и сложных реакций. акие типы сложных реакций вам известны?
16. акие реакции называютс€ цепными? ¬ чем заключаютс€ их особенности?
17. —опоставьте действи€ неорганических катализаторов и ферментов.
18. —толкновение каких молекул, согласно теории јррениуса, приводит к химическому превращению? ¬ли€ют ли на скорость реакции стерические факторы? ќбоснуйте ваш ответ.
19. „ем отличаютс€, согласно теории јррениуса, активные молекулы от неактивных и как можно рассчитать долю активных молекул?
20. ќбъ€сните суть теории активированного комплекса.
21. ѕрименительно к процессам всасывани€ лекарственных препаратов из желудка в кровь и выведени€ их в мочу используют термин Уврем€ полувыведени€Ф. онстанта скорости процесса выведени€ лекарства из организма называетс€ константой элиминации. ќна €вл€етс€ характеристикой препарата и дл€ разных препаратов имеет значени€ 10Ц5 Ц 10Ц3 сЦ1. –ассчитайте врем€ полувыведени€ препарата, если константа элиминации равна 6×10Ц5 сЦ1, а процесс выведени€ протекает по первому пор€дку. (11500 с.)
22. ¬ чем физический смысл пон€ти€ Уэнерги€ активацииФ? ќбъ€сните в рамках теории јррениуса вли€ние температуры на скорость реакции.
23. —корость оседани€ эритроцитов (—ќЁ) описываетс€ кинетическим уравнением 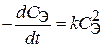 , где
, где 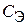 Ц концентраци€ эритроцитов в плазме крови. “ангенс угла наклона пр€мой, построенной в координатах
Ц концентраци€ эритроцитов в плазме крови. “ангенс угла наклона пр€мой, построенной в координатах 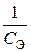 = f (t), равен 10Ц13л/(моль×мин). ќбъ€сните физический смысл этой величины. ѕо какому пор€дку протекает данный процесс?
= f (t), равен 10Ц13л/(моль×мин). ќбъ€сните физический смысл этой величины. ѕо какому пор€дку протекает данный процесс?
24. Ёнерги€ активации дл€ реакции распада пероксида водорода под действием иона железа (II) и молекулы каталазы соответственно равна 42 и 7,1 кƒж/моль. ¬ каком случае разложение H2ќ2 произойдет быстрее и почему?
25. ћожно ли по написанному уравнению химической реакции установить кинетический пор€док реакции?
26. ак вли€ет катализатор на величину энергии активации реакции и на равновесие?
27. акие реакции называютс€ каталитическими? ѕриведите примеры таких реакций в живых организмах.
28. ≈сть ли отличи€ между реакци€ми первого и второго пор€дков?
29. »меют ли отношение фотохимические реакции к механизму действи€ зрительного анализатора? ќтвет обоснуйте.
30. акие реакции называютс€ цепными? ѕриведите примеры.
31. ќбъ€сните физический смысл константы скорости реакции. ќт каких факторов зависит ее величина?
32. онстанта скорости некоторой реакции имеет размерность л/(моль×с). аков пор€док этой реакции? ќтвет обоснуйте.
33. явл€етс€ ли образование кислорода как побочного (но очень важного) продукта фотосинтеза универсальным свойством фотосинтеза? ќтвет обоснуйте.
34. акие теории катализа вам известны? аковы основные положени€ этих теорий?
35. ѕокажите роль фермент-субстратного комплекса в ферментативном катализе.
36. ќбычный путь лекарственных веществ в организме можно рассматривать как последовательность двух процессов: всасывание из желудка в кровь и выведение из крови в мочу. аждый из этих процессов характеризуютс€ константами скоростей. »зобразите кинетические кривые изменени€ массы лекарственного вещества в желудке, крови и моче. ак можно использовать эти зависимости дл€ прогнозировани€ дозы и частоты приема лекарственного вещества?
37. ѕриведите основные стадии процесса фотосинтеза. ¬ чем состоит сущность фотохимической реакции?
38. „то понимают под положительным и отрицательным катализом? ѕриведите примеры.
39. ¬ чем про€вл€етс€ специфичность и избирательность катализаторов? ѕокажите это на конкретных примерах.
40. ќбъ€сните вли€ние pH среды на ферментативную активность.
41. »звестно, что согласно закону Ёйнштейна один поглощенный квант энергии вызывает единственный химический процесс. ¬ то же самое врем€ квантовый выход может быть равен 106. ак объ€снить это кажущеес€ противоречие?
42. ака€ разница между молекул€рностью и кинетическим пор€дком реакции? ћожет ли кинетический пор€док реакции быть равен молекул€рности?
43. ¬о сколько раз скорость реакции разложени€ пероксида водорода под действием каталазы (k 2 = 3,5×107 сЦ1) больше скорости той же реакции, но под действием иона Fe2+ (k 1 = 56,0 сЦ1)? ак будут соотноситьс€ между собой энергии активации данных процессов?
44. ћожно ли предсказать по уравнению реакции зависимость ее скорости от концентрации?
45. акие факторы вли€ют на скорость ферментативной реакции? ¬ чем физический смысл константы ћихаэлиса?
46. ¬ лабораторных услови€х кислород часто получают разложением бертолетовой соли в присутствии оксида марганца (IV) в качестве катализатора. акой это тип катализа - гетерогенный или гомогенный?
47. „то называетс€ квантовым выходом фотохимической реакции?
48. ќбъ€сните возможные изменени€ константы скорости реакции при проведении ее в присутствии катализатора.
49. ѕочему при повышении температуры скорость реакции увеличиваетс€ независимо от того, проходит она с выделением или поглощением теплоты?
50. ∆иры и углеводы окисл€ютс€ в живом организме при температуре около 37 ∞—, а вне его их окисление происходит при 450 Ц 500 ∞—. ќбъ€сните причины этого €влени€.
51. –еакци€ первого пор€дка протекает на 30 % за 60 мин. «а какое врем€ реакци€ завершитс€ (врем€ разложени€ 99,9% вещества)? (1163 мин)
52. –еакци€ ј + ¬ → 2D второго пор€дка. ≈сли начальные значени€ концентрации реагентов равны, то за 300 с реакци€ проходит на 20 %. «а какое врем€ она пройдет на 70 %? (2800 с)
53. –ассчитайте, при какой температуре реакци€ практически закончитс€ через 15 мин, если при 293 на это потребуетс€ 2 ч. “емпературный коэффициент реакции равен 3. (312 )
54. ак изменитс€ скорость элементарной гипотетической реакции M + 3L = 2D, если концентрацию вещества M увеличить в 4 раза, а концентрацию вещества L уменьшить во столько же раз? (”меньшитс€ в 16 раз)
55. —опоставьте константы скорости двух реакций первого пор€дка 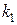 и
и 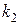 , если период полураспада первой реакции в три раза больше, чем второй. (1:3.)
, если период полураспада первой реакции в три раза больше, чем второй. (1:3.)
56. ѕри авари€х на атомных станци€х часто происходит выброс изотопа йода с массовым числом 131. ѕериод полураспада этого изотопа равен 8 сут. «а какое врем€ произойдет разложение изотопа йода на 90 %? (26,8 сут)
57. –азложение пероксида водорода в водном растворе протекает как реакци€ первого пор€дка с периодом полураспада 13,6 мин. ќпределите врем€, которое потребуетс€ дл€ разложени€ при аналогичных услови€х 90 % пероксида водорода. (46 мин)
58. ѕри 20 ∞— 0,01 ћ раствор уксусноэтилового эфира омыл€етс€ 0,02 ћ раствором NаќH на 10 % в течение 23 мин. ак изменитс€ это врем€, если уменьшить концентрации реагирующих веществ в п€ть раз? (115 мин)
59. —корость бактериального гидролиза мышц рыб удваиваетс€ при переходе от температуры Ц1,1 ∞— к температуре +2,2 ∞—. ќцените энергию активации этой реакции. ≈сть ли здесь кака€-нибудь св€зь с проблемой хранени€ рыбы? (130,08 кƒж/моль)
60. —корость многих реакций удваиваетс€ при повышении температуры на каждые 10 градусов. —чита€, что така€ реакци€ идет при 300 , оцените, какова должна быть энерги€ активации, чтобы эта закономерность выполн€лась. (53,35 кƒж/моль)
61. ƒиссоциаци€ фосгена COCl2 = CO + Cl2 при температуре 109 ∞— протекает с константой скорости 5×10Ц3 минЦ1. акое количество фосгена разложитс€ при данной температуре за 100 мин после начала реакции, если начальна€ концентраци€ фосгена составила 1 моль/л? (0,396 моль)
62. —рок годности некоторого лекарственного препарата при 40 ∞— равен 1 году. ќпределите срок годности его при комнатной температуре (20 ∞C), если считать, что разложение его идет по первому пор€дку и температурный коэффициент равен 2. (4 года)
63. ƒенатураци€ белка (реакци€ 1-го пор€дка) при 60 ∞— прошла за 15,32 мин. на 50 %. ќпределите врем€ его распада на 90 % при этих же услови€х. ћожно ли получить ответ задачи, если в условии вместо периода полураспада, известны энерги€ активации и константа скорости при 20 ∞—? (51 мин.)
64. онстанта разложени€ таблеток анальгина при 20 ∞— равна
1,5×10Ц9 сЦ1. –ассчитайте срок хранени€ таблеток (врем€ разложени€ 10 % вещества) в этих услови€х. (2,23 года)
65. ѕо€вление изотопов азота наблюдаетс€ при €дерных взрывах. ќдин из таких изотопов имеет период полураспада 9,93 ч. ака€ часть данного изотопа распадаетс€ через сутки после начала процесса? (0,81)
66. акой должна быть энерги€ активации, чтобы скорость реакции увеличилась в три раза при повышении температуры от 50 до 60 ∞—? (98,24 кƒж/моль)
67. ¬ сосуде имеетс€ 0,025 г радона. ѕериод полураспада радона 3,82 дн€. акое количество радона (%) останетс€ в сосуде через 2 недели? (8 %)
68. ‘армацевтический препарат этилхлорид вызывает временную анестезию. ѕри гидролизе (в среде 80%-го этилового спирта) обнаружено, что изменение его концентрации во времени происходит следующим образом:
| t, ч | |||||
| C(—2H5Cl), моль/л | 0,312 | 0,258 | 0,186 | 0,085 | 0,047 |
ќпределите константу скорости и период полупревращени€, если она протекает по первому пор€дку. (0,0496 часЦ1, 14 час.)
69. —пазмолитин в водном растворе при 353 разлагаетс€ наполовину за 90 мин. –ассчитайте врем€, за которое спазмолитин разложитс€ наполовину при температуре 293 , если энерги€ активации этого процесса равна 75,7 кƒж/моль. (12,3 дн€)
70. ѕри хранении таблеток анальгина при комнатной температуре (20 ∞—) происходит его разложение с константой скорости 1,5×10Ц9сЦ1. –ассчитайте срок хранени€ таблеток анальгина (врем€ разложени€ 10 % вещества) в этих услови€х и в услови€х хранени€ в холодильнике (Ц5 ∞—), если энерги€ активации процесса разложени€ равна 60 кƒж/моль. (2,23 года; 22,07 года)
71. ѕрепарат " алий-нормин" нормализует количество ионов кали€ в сыворотке крови. ѕосле приема таблеток концентраци€ ионов кали€ в сыворотке крови изменилась следующим образом:
| t, ч | |||||
| C( +), мг /л | 4,60 | 4,40 | 4,20 | 4,08 | 3,53 |
ќпределите константу скорости реакции распада таблеток. (0,0109 часЦ1)
72. ќдним из опасных последствий €дерного взрыва €вл€етс€ образование изотопа 90Sr и его внедрение в кости вместо кальци€. ѕериод полураспада изотопа равен 28,1 года. ѕредположим, что 1 мкг был поглощен новорожденным. —колько останетс€ его а) к 18 годам, б) к 70 годам? (а) 0,64 мкг; б) 0,18 мкг)
73. –еакци€ имеет энергию активации 64,79 кƒж/моль и константу скорости реакции при 20 ∞— равную 1,2 минЦ1. –ассчитайте величину константы скорости при 0 ∞—. (0,17 минЦ1)
74. “емпературный коэффициент скорости некоторой реакции равен 2. ¬ычислите константу скорости реакции при 390 ∞—, если при 362 ∞— она равна 7,5×10Ц4 сЦ1. (5,22Ј10Ц3 сЦ1)
75. ћонохлоруксусна€ кислота при 298 реагирует с водой (вода вз€та в большом избытке). онстанта скорости этой реакции равна 7×10Ц5 сЦ1. ќпределите врем€ полураспада и врем€, в течение которого прореагирует 75 % монохлоруксусной кислоты. (2,74 час; 5,5 час)
76. –азложение пероксида водорода в водном растворе протекает по кинетическому уравнению реакции первого пор€дка с константой скорости 5,081×10Ц2 минЦ1. ќпределите врем€, за которое пероксид водорода разложитс€ на 50 %. (13,6 мин)
77. ƒиссоциаци€ фосгена при температуре 109 ∞— протекает с константой скорости 5×10Ц3 минЦ1, при повышении температуры до 209 ∞— константа скорости становитс€ равной 6,76×10Ц1 минЦ1. –ассчитайте энергию активации процесса диссоциации фосгена и константу скорости реакции при температуре 150 ∞—. (75,14 кƒж/моль; 4,5×10Ц2 минЦ1)
78. ѕри гидролизе 1 кг сахарозы в присутствии воды и фермента гидролиза сахаразы через 5 ч осталось непрореагировавшим 600 г сахарозы. ¬ычислите количество сахарозы, разложившейс€ через 30 мин от начала реакции. (49,4 г.)
79. ¬ услови€х 40-градусной жары новокаин гидролизуетс€ с константой скорости 0,66 чЦ1. Ёнерги€ активации этого процесса равна 55,2 кƒж/моль. —колько процентов новокаина разложитс€ за 30 дней его хранени€ при 40-градусном морозе? (27,85 %)
80. ƒл€ определени€ срока годности порошка папаверина гидрохлорида проведено исследование по методике "ускоренного старени€" и получены следующие результаты:
| врем€, сут | ||||
| (t=50 ∞C) C, % | 99,79 | 99,38 | 98,91 | |
| (t=80 ∞C) C, % | 99,58 | 98,86 | 97,80 |
ќпределите срок годности порошка (врем€ разложени€ 10 % вещества) при температуре хранени€ 20 ∞—. (1,22 года)
81. –еакци€ первого пор€дка при 20 ∞— осуществл€етс€ на 40 % за 50 мин. ѕри какой температуре реакци€ завершитс€ полностью за это же врем€, если энерги€ активации составл€ет 36 кƒж/моль. (357,15 )
82. —одержание лекарственного препарата в растворе при гидролизе за 14 дней уменьшилось на 6,85 %. ќпределите константу скорости процесса, период полупpевpащени€ и срок хранени€ препарата (врем€ разложени€ 10 % вещества). (0,005 днЦ1; 136 дн; 21 день)
83. ѕри хранении таблеток амидопирина было установлено, что при 80 ∞— и 90 ∞— константы скорости разложени€ соответственно равны 1,64×10‑6 сЦ1 и 4,20×10Ц6 сЦ1. ќпределите срок хранени€ таблеток (врем€ разложени€ 10 % вещества) при 20 ∞—. (2,22 года)
84. онстанта скорости гидролиза новокаина при 313 равна 0,66 чЦ1, энерги€ активации реакции - 55,2 кƒж/моль. —колько времени потребуетс€ дл€ разложени€ 50% новокаина при температуре хранени€ 263 ? (59 ч.)
85. ƒл€ реакции разложени€ сульфацила натри€ при 460 константа скорости - 3,5×10Ц2 минЦ1, а при 518 она равна 3,43×10Ц1 минЦ1. ќпределите температурный коэффициент скорости и константу скорости реакции при 486 . (1,48; 9,78Ј10Ц2 минЦ1)
86. “емпературный коэффициент скорости реакции гидролиза 5 % норсульфазола-натри€ равен 3. ќптимальна€ температура хранени€ препарата 20 ∞— ѕри какой температуре нужно проводить "искусственное старение" препарата, чтобы скорость его разрушени€ возросла в 80 раз? (59,8∞—)
87. Ќа основании экспериментальных данных реакции гидролиза сахарозы установлено:






