ѕримеры решени€ задач
«адача 1. –аствор хлорида кали€ с концентрацией, равной 0,2 моль/л, имеет при 298 мол€рную электропроводность 1,24×10Ц2 ќмЦ1м2мольЦ1. –ассчитайте удельную электропроводность этого раствора.
–ешение
”дельна€ и мол€рна€ электропроводности св€заны соотношением
 ,
,
где  - удельна€ электропроводность, ќмЦ1мЦ1,
- удельна€ электропроводность, ќмЦ1мЦ1,
 - мол€рна€ электропроводность, ќмЦ1м2мольЦ1,
- мол€рна€ электропроводность, ќмЦ1м2мольЦ1,
 - концентраци€ раствора, моль/м3.
- концентраци€ раствора, моль/м3.
¬ыразив концентрацию в моль/м3 , найдем c:
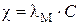 = 1,24×10Ц2×0,2×103 = 2,48 ќмЦ1мЦ1.
= 1,24×10Ц2×0,2×103 = 2,48 ќмЦ1мЦ1.
«адача 2. –ассчитайте мол€рную электропроводность уксусной кислоты при бесконечном разведении, если мол€рные электропроводности при бесконечном разведении сол€ной кислоты, ацетата натри€ и хлорида натри€ соответственно равны 426,1×10Ц4; 91,0×10Ц4 и 126,4×10Ц4 ќмЦ1м2мольЦ1.
–ешение
—огласно закону ольрауша, мол€рна€ электропроводность при бесконечном разведении (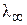 ) равна сумме подвижностей аниона и катиона, вход€щих в состав электролита:
) равна сумме подвижностей аниона и катиона, вход€щих в состав электролита:

следовательно,
 .
.
“аким же образом можно представить данные в задаче мол€рные электропроводности при бесконечном разведении:
 ;
;
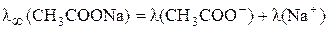 ;
;
 .
.
¬ыразим из этих уравнений подвижности иона водорода, ацетатЦиона и подставим их в выражение дл€  :
:
 ;
;
 ;
;
“огда

 =
=  =
=
= 426,1×10Ц4 + 91,0×10Ц4 Ц 126,4×10Ц4 = 390,7×10Ц4 ќмЦ1м2мольЦ1.
«адача 3. ”дельна€ электропроводность 0,1 н раствора —Ќ3—ќќЌ равна 0,16 ќмЦ1мЦ1, а мол€рна€ электропроводность при бесконечном разведении равна 390,7×10Ц4 ќмЦ1м2мольЦ1. Ќайдите степень и константу диссоциации уксусной кислоты.
–ешение
¬ычислим мол€рную электропроводность раствора уксусной кислоты при данной концентрации:
 .
.
—тепень диссоциации —Ќ3—ќќЌ равна:
 .
.
онстанту диссоциации можно найти по закону ќствальда:
дисс = 
¬ данном случае концентрацию следует выражать в моль/л.

¬опросы и задани€
1. –астворы, как и металлы, обладают способностью проводить электрический ток. ќбъ€сните, что €вл€етс€ носителем электричества в металлах и растворах.
2. проводникам какого рода относ€тс€ расплавы и растворы солей?
3. ќт каких факторов зависит электропроводность растворов?
4. ќбъ€сните суть метода, примен€емого дл€ определени€ электропроводности растворов электролитов.
5. „то такое электропроводность проводника? ак она св€зана с сопротивлением и в каких единицах измер€етс€?
6. ƒл€ каких расчетов используетс€ величина посто€нной сосуда и как она определ€етс€ экспериментально?
7. „то называетс€ удельной электропроводностью раствора и как она определ€етс€?
8. ќбъ€сните зависимость удельной электропроводности сильных и слабых электролитов от концентрации и представьте ее графически.
9. „то называетс€ мол€рной электропроводностью? ак можно ее определить?
10. ќбъ€сните, как измен€етс€ мол€рна€ электропроводность с разведением. ќдинаковы ли эти изменени€ дл€ слабых и сильных электролитов?
11. Ќапишите и по€сните математическое выражение закона ольрауша.
|
|
|
12. „то понимают под абсолютной скоростью движени€ иона и как она определ€етс€? ќбъ€сните разницу между абсолютной скоростью движени€ ионов и их подвижностью.
13. ƒл€ каких целей используетс€ измерение электропроводности растворов?
14. ќбъ€сните сущность и практическое значение метода кондуктометрического титровани€.
15. ”кажите значение измерени€ электропроводности в биологических системах.
16. аким образом используетс€ €вление электропроводности дл€ диагностических исследований и физиотерапевтического лечени€?
17. аковы размерности удельной и мол€рной электропроводности?
18. ак определ€ют мол€рную электропроводность слабых электролитов при бесконечном разведении?
19. ќбъ€сните вид кривой кондуктометрического титровани€ сильной кислоты сильным основанием.
20. ак рассчитывают степень диссоциации слабого электролита по данным об электропроводности его растворов?
21. ак определ€ют мол€рную электропроводность сильных электролитов при бесконечном разведении?
22. ћожно ли вычислить константу электролитической диссоциации по величине электропроводности?
23. ќбъ€сните принцип кондуктометрического титровани€.
24. ћожно ли измерить экспериментально электропроводность раствора?
25. ”дельна€ электропроводность клеток находитс€ в пределах 10Ц3 - 10Ц9 ќмЦ1мЦ1, а межклеточной жидкости больше 10Ц3 ќмЦ1мЦ1. ќбъ€сните причину такого различи€.
26. ќбъ€сните вид кривой кондуктометрического титровани€ слабой кислоты сильным основанием.
27. ћожно ли по величине электропроводности определить концентрацию ионов в растворе?
28. ѕри кислотноЦосновном титровании желудочного сока в присутствии индикатора определение точки эквивалентности затруднено изЦза мутности раствора. ѕредложите другие методы определени€ точки эквивалентности.
29. ¬ каких единицах измер€ютс€ абсолютна€ скорость движени€ иона и его подвижность?
30. ак, использу€ электропроводность раствора слабого электролита, определить степень и константу электролитической диссоциации растворенного вещества?
31. ќбъ€сните причины различи€ подвижностей ионов H+, Na+.
32. ак вли€ет температура на электропроводность растворов и металлов?
33. ¬ычислите посто€нную электродного сосуда, если сопротивление 0,02 н раствора KCl при температуре 20∞— равно 300 ќм, а удельна€ электропроводность 0,25 ќмЦ1мЦ1. (75 мЦ1)
34. ¬ычислите посто€нную электродного сосуда, если сопротивление 0,02 н раствора KCl при температуре 16∞— равно 300 ќм, а удельна€ электропроводность 0,229 ќмЦ1мЦ1. (68,9 мЦ1)
35. ¬ычислите константу диссоциации уксусной кислоты, если степень диссоциации ее в растворе с концентрацией 0,001 ћ равна 0,125. (1,79 10Ц5)
36. —опротивление кров€ной сыворотки, измеренное в сосуде с посто€нной, равной 99,86 мЦ1, при 25 ∞— составл€ет 100 ќм. ќпределите удельную электропроводность сыворотки. (0,9986 ќмЦ1мЦ1)
37. ”дельна€ электропроводность раствора бензойной кислоты равна 2,1×10Ц2 ќмЦ1мЦ1. ќпределите концентрацию раствора, если степень диссоциации бензойной кислоты равна 0,3. ћол€рна€ электропроводность при бесконечном разведении составл€ет 369,9×10Ц4 ќмЦ1м2мольЦ1. (1,89 моль/м3)
38. ѕодвижность ионов NH4+ равна 64×10Ц4 ќмЦ1м2мольЦ1, а ионов ќHЦ- 174×10Ц4 ќмЦ1м2мольЦ1. –ассчитайте удельную электропроводность 0,1 н раствора NH4ќH, степень диссоциации которого 0,01. (0,0238 ќмЦ1мЦ1)
|
|
|
39. Ѕудут ли отличатьс€ значени€ удельной и эквивалентной электропроводности дл€ 0,01 ћ растворов: а) HCl и KCl, б) CH3COOH и CH3COOK?
40. ћол€рные электропроводности при бесконечном разведении NaCl, NaOH и ќЌ равны соответственно 109×10Ц4; 217,5×10Ц4 и 238×10Ц4 ќмЦ1м2мольЦ1. Ќайдите подвижности ионов Na+ и ClЦ, если подвижность ионов + равна 64,6×10Ц4 ќмЦ1м2мольЦ1. (44,1×10Ц4 ќмЦ1м2мольЦ1; 64,51×10Ц4 ќмЦ1м2мольЦ1)
41. ”дельна€ электропроводность 0,001 н раствора KNO3 равна  ќмЦ1мЦ1, подвижности ионов + и NO3Ц- соответственно 64,6×10Ц4 и 62×10Ц4 ќмЦ1м2мольЦ1. ќпределите мол€рную электропроводность раствора и кажущуюс€ степень диссоциации KNO3. (0,0125 ќмЦ1м2мольЦ1; 0,987)
ќмЦ1мЦ1, подвижности ионов + и NO3Ц- соответственно 64,6×10Ц4 и 62×10Ц4 ќмЦ1м2мольЦ1. ќпределите мол€рную электропроводность раствора и кажущуюс€ степень диссоциации KNO3. (0,0125 ќмЦ1м2мольЦ1; 0,987)
42. ”дельна€ электропроводность 0,0001 н полностью диссоцииро≠ванного раствора KCl равна 1,3×10Ц3 ќмЦ1мЦ1, подвижность ионов + Ц
64,6×10Ц4 ќмЦ1м2мольЦ1. ¬ычислите абсолютную скорость движени€ ионов ClЦ. (6,78Ј10Ц8 м2¬Ц1сЦ1)
43. ”дельна€ электропроводность 0,5 н раствора одноосновной слабой кислоты при 25∞— равна 0,09 ќмЦ1мЦ1. ћол€рна€ электропроводность при бесконечном разведении составл€ет 345×10Ц4 ќмЦ1м2мольЦ1. ¬ычислите степень диссоциации и константу диссоциации. (0,0052; 1,36Ј10Ц5)
44. ѕри 18∞— абсолютна€ скорость движени€ ионов цези€ и хлора соответственно равна 6,98×10Ц8 и 6,76×10Ц8 м2сЦ1¬Ц1. ”дельна€ электропропроводность хлорида цези€ при разведении 1000 л/моль составл€ет 1,306×10Ц2  . ¬ычислите кажущуюс€ степень диссоциации этой соли в данном растворе. (0,98)
. ¬ычислите кажущуюс€ степень диссоциации этой соли в данном растворе. (0,98)
45. ѕри 25 ∞— константа диссоциации угольной кислоты по I ступени равна 4,5×10Ц7 моль/л. ѕодвижность ионов Ќ+ и Ќ—ќ3Цпри этой температуре - соответственно 349,8×10Ц4 и 44,5×10Ц4 ќмЦ1м2мольЦ1. ћол€рна€ электропроводность раствора некоторой концентрации равна 2,6×10Ц4 ќмЦ1м2мольЦ1. –ассчитайте концентрацию кислоты. (1,035Ј10Ц2 моль/л)
46. ѕри титровании 100 мл сол€ной кислоты раствором гидроксида натри€ концентрации 1,045 н получены следующие значени€ электропроводности:
| ќбъем NaOH, мл | ||||||
| c×102, ќмЦ1мЦ1 | 3,90 | 2,84 | 1,77 | 1,24 | 2,07 | 2,94 |
ќпределите концентрацию кислоты в моль/л.
47. ”дельна€ электропроводность 0,05 н раствора уксусной кислоты при 18 ∞— равна 3,24×10Ц2 ќмЦ1мЦ1. ћол€рна€ электропроводность при бесконечном разведении ацетата натри€ составл€ет 77,5×10Ц4 ќмЦ1м2мольЦ1, а подвижность ионов натри€ и водорода Ц соответственно 43,5×10Ц4 и 315×10Ц4  . ¬ычислите концентрацию ионов водорода в этом растворе и константу диссоциации уксусной кислоты. (9,28Ј10Ц4моль/л; 1,76Ј10Ц5)
. ¬ычислите концентрацию ионов водорода в этом растворе и константу диссоциации уксусной кислоты. (9,28Ј10Ц4моль/л; 1,76Ј10Ц5)
48. Ќайдите концентрацию раствора электролита, если степень диссоциации равна 0,6, а константа диссоциации - 9×10Ц3. (0,01 моль/л)
49. —опротивление 0,1 н раствора AgNO3 при 25 ∞— равно 1090 ќм, сопротивление 0,02 н KCl в том же сосуде при тех же услови€х - 4318 ќм, а удельна€ электропроводность его составл€ет 0,277 ќмЦ1мЦ1. ¬ычислите удельную и мол€рную электропроводности раствора AgNO3. (1,097 ќмЦ1мЦ1; 109,7Ј10Ц4 ќмЦ1м2мольЦ1)
50. –аствор, содержащий 16 г CuSO4 в 1 л, находитс€ между двум€ электродами с S=4 см2 на рассто€нии 0,7 см. —опротивление раствора равно 23 ќм. Ќайдите удельную и мол€рную электропроводности раствора сульфата меди. (0,76 ќмЦ1мЦ1; 76,1Ј10Ц4 ќмЦ1м2мольЦ1)
51. ѕри титровании 100 мл маринадной заливки 0,5н раствором NaOH получены следующие данные:
| ќбъем NaOH, мл | 8,0 | 9,0 | 10,0 | 11,0 | 13,0 | 13,5 | 14,0 |
| R, ќм | 75,0 | 71,4 | 68,9 | 66,6 | 62,5 | 55,6 | 41,6 |
ќпределите нормальную концентрацию уксусной кислоты в заливке.
52. ”дельна€ электропроводность 0,01 н —H3—ќќH при 25 ∞— равна 1,56×10Ц2 ќмЦ1мЦ1. ћол€рна€ электропроводность при бесконечном разведении составл€ет 390,7×10Ц4 ќмЦ1м2мольЦ1. ¬ычислите pH раствора. (3,4)
53. –ассчитайте мол€рную электропроводность гидроксида аммони€ при бесконечном разведении, если мол€рные электропроводности при бесконечном разведении гидроксида натри€, хлорида аммони€ и хлорида натри€ соответственно равны 248,4×10Ц4, 149,85×10Ц4 и 126,4×10Ц4 ќмЦ1м2мольЦ1. (271,85Ј10Ц4 ќмЦ1м2мольЦ1)
|
|
|
54. ”дельна€ электропроводность 0,06 н раствора уксусной кислоты при 25 ∞— равна 3,97×10Ц2 ќмЦ1мЦ1. ћол€рна€ электропроводность при бесконечном разведении ацетата натри€ составл€ет 91×10Ц4 ќмЦ1м2мольЦ1, а подвижность ионов натри€ и водорода - соответственно 50,1×10Ц4 и 349,8×10Ц4 ќмЦ1м2мольЦ1. ¬ычислить концентрацию ионов водорода в этом растворе и константу диссоциации уксусной кислоты. (1,02Ј10Ц3 моль/л; 1,73Ј10Ц5)
55. –ассчитайте концентрацию KCl в растворе, если удельна€ электропроводность раствора равна 0,277 ќмЦ1мЦ1, а мол€рна€ электропроводность - 136,3×10Ц4 ќмЦ1м2мольЦ1. (0,02 моль/л)
56. ќпределите степень электролитической диссоциации муравьиной кислоты в растворе с концентрацией 0,05 н, а также мол€рную электропроводность при 25 ∞—, если константа диссоциации муравьиной кислоты 1,77×10Ц4, а подвижности ионов Ќ+ и Ќ—ќќЦ равны соответственно 349,8×10Ц4 и 54,6×10Ц4 ќмЦ1м2мольЦ1. (0,06; 24,26Ј10 Ц4 ќмЦ1м2мольЦ1)
57. ƒл€ определени€ концентрации щавелевой кислоты в отбеливателе используют зависимость удельной электропроводности (c) от концентрации (C) кислоты в растворе:
| C, моль/л | 0,004 | 0,007 | 0,015 | 0,030 | 0,060 | 0,121 | 0,243 |
| χ×102, ќмЦ1мЦ1 | 2,5 | 3,8 | 5,00 | 8,00 | 12,3 | 21,00 | 36,3 |
ѕостройте график в координатах lg(c) - f(lgC) и определите концентрацию раствора, если удельна€ электропроводность его равна 14,4×10Ц2 ќмЦ1мЦ1.
58. ¬ сосуд дл€ измерени€ электропроводности помещены круглые платиновые электроды диаметром 1,34 см, рассто€ние между ними 1,72 см. —осуд заполнен 0,05 н раствором NaNO3. ѕри напр€жении 0,5 ¬ через раствор проходит ток силой 1,85 мј. –ассчитайте удельную и мол€рную электропроводности раствора. (0,45 ќмЦ1мЦ1; 90,0Ј10Ц4 ќмЦ1м2мольЦ1)
59. “аблетки ацидин Ц пепсина при введении в желудок легко гидролизуютс€, выдел€€ свободную сол€ную кислоту. ѕри титровании 25 мл раствора, полученного при гидролизе 0,25 н раствором гидроксида кали€, были получены следующие результаты:
| ќбъем OH, мл | 3,2 | 6,1 | 9,2 | 15,4 | 20,1 | 23,4 |
| c×102, ќмЦ1мЦ1 | 3,28 | 2,56 | 1,85 | 1,64 | 2,40 | 2,96 |
ќпределите концентрацию сол€ной кислоты в моль/л.
60. ѕри 25∞— мол€рные электропроводности при бесконечном разведении растворов хлорацетата натри€, хлорида натри€ и сол€ной кислоты соответственно равны 90×10Ц4; 126,4×10Ц4 и 426×10Ц4 ќмЦ1м2мольЦ1. ¬ычислите значение мол€рной электропроводности при бесконечном разведении раствора хлоруксусной кислоты. (389,6Ј10Ц4 ќмЦ1м2мольЦ1)
61. ѕри кондуктометрическом титровании 25 мл раствора пепсина 0,1 ћ раствором гидроксида кали€ были получены следующие результаты:
| V ќH, мл | 3,2 | 6,0 | 9,2 | 15,6 | 20,0 | 23,5 |
| c×102, ќмЦ1мЦ1 | 3,20 | 2,56 | 1,86 | 1,64 | 2,38 | 2,96 |
ќпределите концентрацию пепсина в моль/л.
62. јбсолютные скорости движени€ ионов цези€ и хлора при 25 ∞— равны 6,98×10Ц8 и 6,76×10Ц8 м2/с׬. ”дельна€ электропроводность хлорида цези€ при разбавлении 500 л/моль составл€ет 2,612×10Ц2 ќмЦ1мЦ1. ¬ычислите степень диссоциации хлорида цези€ в данном растворе. (0,985)
63. —опротивление водной выт€жки озоленного образца сыра, содержащего поваренную соль, в €чейке с электродами площадью 2,54 см2 и рассто€нием между ними 0,65 см равно 5,61 ќм. ќпределите процентное содержание поваренной соли в сыре, если мол€рна€ электропроводность раствора Ц 79,7×10Ц4 ќмЦ1м2мольЦ1, а плотность раствора - 1,01 г/см3. (3,3%)
|
|
|
64. ƒл€ раствора иодата кали€ с концентрацией, равной 1,83×10Ц4 моль/л, мол€рна€ электропроводность равна 113,07×10Ц4 ќмЦ1м2мольЦ1. Ќайдите удельную электропроводность этого раствора. (20,7Ј10Ц4 ќмЦ1мЦ1)
65. ќпределите степень и константу электролитической диссоциации 0,01 н раствора уксусной кислоты, мол€рна€ электропроводность которого при 18 ∞— равна 14,3×10Ц4 ќмЦ1м2мольЦ1. ћол€рна€ электропроводность при бесконечном разведении Ц 349×10Ц4 ќмЦ1м2мольЦ1. (0,041; 1,75Ј10Ц5)
66. ¬ычислите pH 0,1 н раствора NH4OH, если мол€рна€ электропроводность равна 4×10Ц4 ќмЦ1м2мольЦ1, а мол€рна€ электропроводность при бесконечном разведении 272,2×10Ц4 ќмЦ1м2мольЦ1. (11,17)
67. ѕосто€нна€ сосуда дл€ определени€ сопротивлени€ растворов равна 426,4 мЦ1. ¬ычислите сопротивление 0,2 н раствора нитрата кали€, измеренное в этом сосуде при 18 ∞—. ћол€рна€ электропроводность этого раствора равна 98,7×10Ц4 ќмЦ1м2мольЦ1. (216 ќм)
68. ѕодвижности ионов H+ и ќЌЦ при бесконечном разведении равны соответственно 349,8×10Ц4 и 198,5×10Ц4 ќмЦ1м2мольЦ1. ¬ычислите абсолютные скорости движени€ ионов H+ и ќHЦ. (36,2Ј10Ц8 м2¬Ц1сЦ1; 20,57Ј10Ц8 м2¬Ц1сЦ1)
69. ”дельна€ электропроводность 0,03 н —H3—ќќH при 25 ∞— равна 2,81×10Ц2 ќмЦ1мЦ1. ћол€рна€ электропроводность при бесконечном разведении равна 390,7×10Ц4 ќмЦ1м2мольЦ1. ¬ычислите pH этого раствора. (3,14)
70. ¬ычислите степень и константу электролитической диссоциации 0,05 н раствора уксусной кислоты, удельна€ электропроводность которого при 18 ∞— равна 3,25×10Ц2 ќмЦ1мЦ1. ѕодвижности ионов —Ќ3—ќќЦ и Ќ+ при данной температуре равны соответственно 34×10Ц4 и 315×10Ц4 ќмЦ1м2мольЦ1. (0,0186; 1,73Ј10Ц5)
71. ажуща€с€ степень диссоциации NH4OH при концентрации водного раствора 0,5 н равна 0,0482, а удельна€ электропроводность 0,655 ќмЦ1мЦ1. ќпределите мол€рную электропроводность гидроксида аммони€ при бесконечном разведении. (271,8Ј10Ц4 ќмЦ1м2мольЦ1)
72. —опротивление 0,1 ћ раствора хлорида кали€ равно 35,2 ќм. »змеренное в том же сосуде сопротивление 0,1 ћ раствора AgNO3 составило 42,4 ќм. –ассчитайте удельную и мол€рную электропроводности раствора AgNO3, если дл€ раствора K—l величина удельной электропроводности равна 1,29 ќмЦ1мЦ1. (1,071 ќмЦ1мЦ1; 107,1Ј10Ц4 ќмЦ1м2мольЦ1)
73. онстанта диссоциации азотистой кислоты при 12,5 ∞— равна 4,6×10Ц4. ¬ычислите степень электролитической диссоциации 0,05 н раствора азотистой кислоты. (0,096)
74. —опротивление 0,307 н раствора нитрата серебра при 18 ∞— равно 404,3 ќм. »змеренное в том же сосуде, сопротивление 0,02 н раствора хлорида кали€ составило 4318 ќм, а его удельна€ электропроводность - 0,239 ќмЦ1мЦ1. ¬ычислите удельную и мол€рную электропроводности раствора нитрата серебра. (2,55 ќмЦ1мЦ1; 83,15Ј10Ц4 ќмЦ1м2мольЦ1)
75. ѕри 25∞— константа диссоциации масл€ной кислоты равна 1,5×10Ц5. ћол€рна€ электропроводность раствора этой кислоты при концентрации 15,6×10Ц3 моль/л составл€ет 11,6×10Ц4 ќмЦ1м2мольЦ1. ¬ычислите значение мол€рной электропроводности при бесконечном разведении масл€ной кислоты. (374,2Ј10Ц4 ќмЦ1м2мольЦ1)
76. ѕрепарат "Ѕетацид" легко гидролизуетс€ с выделением свободной сол€ной кислоты. ѕри анализе препарата концентраци€ кислоты определ€етс€ кондуктометрическим титрованием. ѕри титровании 50 мл раствора препарата раствором 0,5 н ќЌ получены следующие данные:
| ќбъем KOH, мл | 3,0 | 6,4 | 9,0 | 17,0 | 20,5 | 25,0 |
| c×102, ќмЦ1мЦ1 | 3,65 | 2,75 | 2,05 | 1,70 | 2,55 | 3,65 |
ќпределите концентрацию сол€ной кислоты в моль/л.
77. ¬ычислите посто€нную электродного сосуда, если сопротивление 0,02 н раствора некоторого электролита при температуре 25 ∞— равно 350 ќм, а удельна€ электропроводность Ч 0,2765 ќмЦ1мЦ1. (96,77 мЦ1)
78. ¬ычислите посто€нную электродного сосуда, если сопротивление 0,02 н раствора KCl при температуре 22 ∞— равно 300 ќм, а удельна€ электропроводность Ч 0,2606 ќмЦ1мЦ1. (78,18 мЦ1)
79. ажуща€с€ степень диссоциации хлорида лити€ при 25 ∞— в 0,01 н растворе составл€ет 94%. ћол€рна€ электропроводность хлорида лити€ при бесконечном разведении равна 115×10Ц4 ќмЦ1м2мольЦ1. ¬ычислите удельную электропроводность данного раствора. (0,1081 ќмЦ1мЦ1)
80. ѕодвижности ионов Na+, Rb+ и ClЦравны соответственно 50,1×10Ц4; 77,8×10Ц4 и 76,3×10Ц4 ќмЦ1м2мольЦ1. ќбъ€сните причины различи€ мол€рных электропроводностей при бесконечном разведении растворов хлорида натри€ и хлорида рубиди€.
|
|
|
81. ѕредельные значени€ эквивалентной электропроводности ионов Na+, Fe2+, ClЦ, SO42Цсоответственно равны: 50,1Ј10Ц4; 53,5Ј10Ц4; 76,35Ј10Ц4 и 80,0Ј10Ц4 ќмЦ1м2мольЦ1. –асположите в пор€дке возрастани€ значени€ предельной эквивалентной электропроводности водных растворов хлоридов и сульфатов натри€ и железа (II).
82. аков общий вид графической зависимости удельной и мол€рной электропроводности от концентрации растворов сол€ной и уксусной кислот в воде?
83. ¬ чем отличи€ предельных подвижностей ионов Na+ и ClЦ, H+ и OHЦ.
84. ¬ чем заключаетс€ удобство введени€ величины посто€нной сосуда Z? акой физический смысл имеет эта величина?
85. аким образом можно рассчитать степень диссоциации, константу диссоциации, рЌ раствора, использу€ данные определени€ электропроводности?
86. ¬ычислите удельную электропроводность спинномозговой жидкости, если сопротивление 0,1 н раствора KCl при температуре 20∞— равно 24 ќм, а его удельна€ электропроводность 1,167 ќмЦ1мЦ1. —опротивление спинномозговой жидкости, измеренное в том же сосуде, равно 45 ќм. (0,6224 ќмЦ1мЦ1)
87. ”дельна€ электропроводность раствора HCl в желудочном соке при 25∞— равна 0,185 ќмЦ1мЦ1, а его мол€рна€ электропроводность при той же температуре 370,0Ј10Ц4 ќмЦ1м2мольЦ1. ќпределите рЌ желудочного сока. (2,3)
88. ”дельна€ электропроводность 1% Ц го раствора хлорида натри€ (ρ = 1,0053 г/мл) равна 1,8 ќмЦ1мЦ1. –ассчитайте мол€рную электропроводность этого раствора. (105,9Ј10Ц4 ќмЦ1м2мольЦ1)
| ¬арианты индивидуальных заданий дл€ контрольной работы по теме: УЁлектродные потенциалы и электродвижущие силыФ | Ќомера вопросов | ѕоследн€€ цифра | 31, 42, 43, 54, 71 | 1, 6, 51, 58, 88 | 26, 27, 49, 67, 84 | 11, 23, 65, 74, 81 | 8, 14, 25, 55, 80 | 16, 24, 45, 63, 76 | 3, 4, 18, 64, 73 | 7, 13, 32, 59, 76 | 24, 29, 43, 60, 86 | 11, 19, 41, 53, 78 | |
| 23, 41, 42, 87, 88 | 21,40, 57, 74, 84 | 25, 29, 31, 66, 81 | 16, 20, 37, 64, 80 | 5, 15, 22, 56, 76 | 11, 19, 42, 68, 69 | 1, 15, 39, 62, 72 | 2,,24, 27 58, 60 | 19, 33, 38, 53, 85 | 15, 42, 74, 77, 81 | ||||
| 20, 33, 38, 82, 84 | 36, 37, 43, 67, 81 | 22, 23, 38, 65, 80 | 17, 30, 42, 76, 79 | 3, 26, 35, 63, 69 | 6, 41, 50, 62, 70 | 6, 10, 13, 60, 70 | 19, 29, 43, 53, 57 | 16, 41, 67, 74, 78 | 14, 37, 45, 75, 84 | ||||
| 16, 17, 29, 79, 81 | 30, 35, 42, 66, 80 | 20, 34, 41, 64, 76 | 12, 26, 33, 56, 69 | 1, 21, 18, 62, 89 | 38, 49, 51, 60, 72 | 7, 19, 27, 53, 68 | 38, 42, 55, 74, 80 | 11, 37, 42, 77, 79 | 10, 30, 48, 73, 90 | ||||
| 11, 12, 27, 67, 80 | 18, 26, 33, 65, 76 | 17, 32, 42, 63, 69 | 9, 25, 43, 62, 67 | 34, 36, 51, 60, 82 | 29, 31, 39, 53, 73 | 2, 29, 61, 65, 74 | 33, 37, 41, 54, 81 | 15, 30, 45, 75, 82 | 6, 25, 35, 71, 72 | ||||
| 6, 9, 13, 66, 76 | 15, 16, 25, 64, 69 | 12, 24, 45, 56, 62 | 8, 22, 34, 60, 64 | 32, 39, 40, 53, 79 | 23, 27, 28, 71, 86 | 37, 38, 43, 59, 66 | 16, 30, 42, 84, 86 | 10, 25, 48, 73, 87 | 2, 18, 22, 56, 70 | ||||
| 8, 34, 51, 65, 69 | 11, 14, 22, 62, 63 | 9, 19, 48, 60, 79 | 5, 21, 32, 53, 81 | 24, 28, 46, 67, 73 | 4, 13, 20, 85, 87 | 30, 41, 42, 58, 67 | 11, 25, 45, 85, 88 | 6, 14, 22, 53, 72 | 13, 21, 43, 63, 68 | ||||
| 5, 32, 39, 62, 64 | 6, 10, 21, 56, 60 | 4, 8, 53, 82, 83 | 3, 24, 61, 74, 88 | 19, 50, 52, 66, 72 | 2, 17, 37, 69, 78 | 14, 25, 33, 57, 79 | 15, 22, 48, 71, 78 | 7, 21, 35, 60, 70 | 33, 34, 36, 61, 64 | ||||
| 3, 24, 28, 60, 63 | 7, 48, 50, 53, 71 | 5, 28, 52, 55, 87 | 1, 19, 37, 59, 84 | 4, 47, 49, 65, 70 | 7, 12, 30, 76, 77 | 16, 22, 45, 55, 82 | 6, 10, 21, 56, 77 | 2, 13, 34, 62, 68 | 27, 32, 42, 59, 65 | ||||
| 1, 4, 19, 53, 56 | 2, 45, 49, 55, 87 | 3, 39, 47, 59, 88 | 13, 30, 50, 58, 71 | 6, 31, 64, 68, 83 | 9, 10, 74, 75, 80 | 11, 21, 48, 54, 87 | 5, 14, 51, 63, 75 | 18, 27, 32, 61, 69 | 7, 16, 29, 58, 66 | ||||
| ѕред- | послед-н€€ | цифра |






