ƒо третьоњ анал≥тичноњ групи в≥днос€ть кат≥они Ba2+, Sr2+, Ca2+, а також де€кою м≥рою кат≥они Pb2+.
≈лектронна будова кат≥он≥в ц≥Їњ групи: Ba2+ − [Xe]; Sr2+ − [Kr];
Ca2+ − [Ar].
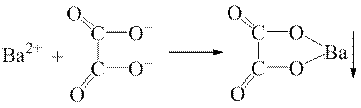
√руповий реагент Ц сульфатна кислота з с(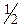 H2SO4)=2 моль/дм3 Ц з кат≥онами третьоњ анал≥тичноњ групи утворюЇ малорозчинн≥ сульфати:
H2SO4)=2 моль/дм3 Ц з кат≥онами третьоњ анал≥тичноњ групи утворюЇ малорозчинн≥ сульфати:
ƒ–(BaSO4)=1,1Ј10Ц10; ƒ–(SrSO4)=3,2Ј10Ц7;
ƒ–(—aSO4)=2,5Ј10Ц5; ƒ–(PbSO4)=1,6Ј10Ц8.
ќск≥льки ц≥ сол≥ утворен≥ сильною кислотою та досить сильними основами (за вин€тком Pb(OH)2), то практично вони не г≥дрол≥зуютьс€. ¬с≥ ц≥ осади б≥лого кольору.
як видно з добутк≥в розчинност≥, найб≥льшу розчинн≥сть маЇ —aSO4. ¬насл≥док цього осадженн€ йон≥в Ca2+ розведеною сульфатною кислотою в≥дбуваЇтьс€ не повн≥стю. „астина йон≥в Ca2+ залишаЇтьс€ у розчин≥. “ому п≥сл€ осадженн€ кат≥он≥в третьоњ анал≥тичноњ групи груповим реагентом необх≥дно провести реакц≥њ ви€вленн€ йон≥в Ca2+ у розчин≥ над осадом. ўоб повн≥стю осадити з розчину йони Ca2+, осадженн€ кат≥он≥в ц≥Їњ анал≥тичноњ групи краще проводити сум≥шшю сульфатноњ кислоти з етанолом.
« п≥двищенн€м температури розчинн≥сть сульфат≥в BaSO4, SrSO4, —aSO4 зм≥нюЇтьс€ мало. Ѕ≥льш повне осадженн€ в≥дбуваЇтьс€ при сто€нн≥ прот€гом 20 хвилин. «азначен≥ сульфати не т≥льки малорозчинн≥ у вод≥, але також нерозчинн≥ в сульфатн≥й та н≥тратн≥й кислотах за вин€тком BaSO4, €кий розчин€Їтьс€ в концентрован≥й H2SO4.
–озчинн≥сть кальц≥й сульфату у значн≥й м≥р≥ п≥двищуЇтьс€ у присутност≥ амон≥й сульфату. ѕри цьому утворюЇтьс€ дуже нест≥йка комплексна с≥ль (NH4)2[—a(SO4)2]. ” присутност≥ етанолу або ацетону розчинн≥сть —aSO4 сильно знижуЇтьс€. якщо осадженн€ кат≥он≥в Ca2+ сульфат-≥онами проводити у водному розчин≥ з додаванн€м до нього 50-60% спирту або ацетону, то вони при цьому повн≥стю перейдуть в осад —aSO4. ÷€ властив≥сть використовуЇтьс€ при в≥дд≥ленн≥ ≥ ви€вленн≥ дробним методом йон≥в Ca2+.
« кат≥онами Ba2+, Sr2+, Ca2+, Pb2+ розчинн≥ сол≥ карбонатноњ кислоти утворюють дуже малорозчинн≥ карбонати б≥лого кольору:
ƒ–(Ba—O3)=4,0Ј10Ц10; ƒ–(Sr—O3)=1,1Ј10Ц10;
ƒ–(—a—O3)=3,8Ј10Ц9; ƒ–(Pb—O3)=7,5Ј10Ц15.
ќднак ц≥ карбонати, на в≥дм≥ну в≥д в≥дпов≥дних сульфат≥в, легко розчин€ютьс€ в кислотах Ц HCl, HNO3, CH3COOH.
—a—O3 розчин€Їтьс€ у надлишку карбонатноњ кислоти:
—a—O3 + —ќ2 + Ќ2ќ → —а(Ќ—ќ3)2.
÷ей процес широко розповсюджений у природ≥ ≥ спричин€Ї утворенн€ жорстких вод, що м≥ст€ть розчинний кальц≥й г≥дрокарбонат.
4.3.1. –еакц≥њ ви€вленн€ кат≥он≥в Ba2+
—пециф≥чних реакц≥й на кат≥они Ba2+ не ≥снуЇ.
Ќайб≥льш характерними реакц≥€ми на кат≥они Ba2+ Ї наступн≥:
ƒосл≥д 1. ’ромат-≥они з кат≥онами Ba2+ утворюють малорозчинний BaCrO4 жовтого кольору:
Ba2+ + CrO42Ц → BaCrO4↓; ƒ–(BaCrO4)=2,3Ј10Ц10,
€кий нерозчинний в оцтов≥й кислот≥, але розчинний в сильних кислотах (HCl, HNO3) внасл≥док того, що в сильнокислому середовищ≥ хромат-≥они перетворюютьс€ в дихромат-≥они, а BaCr2O7, SrCr2O7, CaCr2O7 Ї водорозчинними.
ƒосл≥д 2. ѕри д≥њ на йони Ba2+ дихромат-≥онами в нейтральному або слабкокислому середовищ≥ утворюЇтьс€ малорозчинний жовтий осад бар≥й хромату:
|
|
|
Cr2O72Ц + Ќ2ќ  2CrO42Ц +2Ќ+;
2CrO42Ц +2Ќ+;
CrO42Ц + Ba2+ → BaCrO4↓.
” присутност≥ йон≥в Sr2+ та Ca2+ дану реакц≥ю провод€ть у оцтовокислому середовищ≥ при рЌ=3-5. ¬ипад≥нню осаду BaCrO4 спри€Ї нагр≥ванн€. ат≥они Pb2+ та ≥нш≥, що утворюють з хромат-≥онами осади, заважають проведенню ц≥Їњ реакц≥њ.
ат≥они Sr2+ та Ca2+ також утворюють жовт≥ осади SrCrO4, CaCrO4 при д≥њ на ц≥ кат≥они розчином, що м≥стить йони Cr2O72Ц в аналог≥чних умовах, але утворен≥ хромати —тронц≥ю та альц≥ю розчин€ютьс€ в оцтов≥й кислот≥. ÷€ особлив≥сть використовуЇтьс€ дл€ в≥дд≥ленн€ йон≥в Ba2+ в≥д йон≥в Sr2+ та Ca2+.
ƒосл≥д 3. јмон≥й оксалат (NH4)2C2O4 утворюЇ з йонами Ba2+ б≥лий осад BaC2O4, €кий розчин€Їтьс€ в розчинах HCl, HNO3 та при кипТ€т≥нн≥ Ц у —Ќ3—ќќЌ:
ƒосл≥д 4. Ќатр≥й г≥дрофосфат Na2HPO4 утворюЇ з йонами Ba2+ б≥л≥ осади, розчинн≥ у HCl, HNO3 та —Ќ3—ќќЌ: у нейтральному середовищ≥ Ц кислу с≥ль:
Ba2+ + HPO42Ц  BaHPO4↓;
BaHPO4↓;
а в лужному середовищ≥ Ц середн≥й фосфат:
3Ba2+ + 2HPO42Ц + 2ќЌЦ  Ba3(PO4)2↓ + 2Ќ2ќ.
Ba3(PO4)2↓ + 2Ќ2ќ.
ƒосл≥д 5. ѕ≥рох≥м≥чний анал≥з. Ћетк≥ сол≥ Ѕар≥ю забарвлюють безбарвне полумТ€ пальника в жовтувато-зелений кол≥р.
4.3.2. –еакц≥њ ви€вленн€ кат≥он≥в Sr2+
—пециф≥чних реакц≥й на кат≥они Sr2+ не ≥снуЇ.
ƒосл≥д 1. ƒл€ ви€вленн€ кат≥он≥в Sr2+, зазвичай, використовують г≥псову воду Ц насичений розчин кальц≥й сульфату. ќск≥льки розчинн≥сть стронц≥й сульфату набагато нижча розчинност≥ кальц≥й сульфату, то в≥дбуваЇтьс€ реакц≥€:
CaSO4 + Sr2+ → SrSO4↓ + Ca2+.
ѕри додаванн≥ до розчину, що м≥стить йони Sr2+, г≥псовоњ води ≥ нагр≥ванн≥ пов≥льно утворюЇтьс€ б≥лий осад стронц≥й сульфату. ÷€ реакц≥€ можлива лише за в≥дсутност≥ у досл≥джуваному розчин≥ йон≥в Ba2+, а також ≥нших йон≥в, €к≥ утворюють ≥з сульфат-≥оном малорозчинн≥ сполуки.
ƒосл≥д 2. ѕри д≥њ на йони Sr2+ оксалат-≥он≥в у нейтральному середовищ≥ утворюЇтьс€ осад б≥лого кольору стронц≥й оксалату:
C2O42Ц + Sr2+ → SrC2O4↓,
€кий добре розчин€Їтьс€ в хлоридн≥й, н≥тратн≥й, оцтов≥й кислотах.
ƒосл≥д 3. ѕри внесенн≥ летких солей —тронц≥ю в безбарвне полумТ€ пальника воно забарвлюЇтьс€ у карм≥ново-червоний кол≥р. ÷€ реакц≥€ дуже чутлива, але через високу летк≥сть солей —тронц≥ю забарвленн€ полумТ€ короткочасне, а при малих концентрац≥€х солей —тронц≥ю нав≥ть не про€вл€Їтьс€.
¬и€вленн€ кат≥он≥в Sr2+ найб≥льш складне у пор≥вн€нн≥ ≥з ви€вленн€м ≥нших елемент≥в.
4.3.3. –еакц≥њ ви€вленн€ кат≥он≥в —а2+
—пециф≥чних реакц≥й дл€ ви€вленн€ йон≥в —a2+ не ≥снуЇ.
ƒосл≥д 1. …они —a2+ з ан≥оном щавлевоњ кислоти в близькому до нейтрального середовищ≥ утворюють дуже малорозчинний осад б≥лого кольору кальц≥й оксалату:
CaCl2 + (NH4)2C2O4 → CaC2O4↓ + 2NH4Cl;
Ca2+ + C2O42Ц → CaC2O4↓.
ƒл€ проведенн€ ц≥Їњ реакц≥њ у проб≥рку внос€ть дек≥лька крапель розчину сол≥ альц≥ю, додають краплю розчину оцтовоњ кислоти, пот≥м внос€ть дек≥лька крапель розчину амон≥й оксалату чи ≥ншоњ розчинноњ сол≥ щавлевоњ кислоти, додають 1-2 крапл≥ розчину амон≥аку. альц≥й оксалат Ї найменш розчинною у вод≥ с≥ллю альц≥ю, але вона добре розчин€Їтьс€ в кислотах, за вин€тком —Ќ3—ќќЌ.
ат≥они Ba2+ та Sr2+ теж утворюють з оксалат-≥онами малорозчинн≥ бар≥й оксалат та стронц≥й оксалат, тому у њх присутност≥ в розчин≥ ви€вити йони —а2+ за ц≥Їю реакц≥Їю неможливо.
|
|
|
ƒобутки розчинност≥ оксалат≥в кат≥он≥в третьоњ анал≥тичноњ групи наступн≥:
ƒ–(Ba—2O4)=1,6Ј10Ц7; ƒ–(Sr—2O4)=5,6Ј10Ц8; ƒ–(—a—2O4)=2,57Ј10Ц9.
ќтже, кальц≥й оксалат практично нерозчинний у вод≥, а також у оцтов≥й кислот≥. ќксалати Ѕар≥ю та —тронц≥ю в оцтов≥й кислот≥ розчин€ютьс€, причому Ba—2O4 розчин€Їтьс€ краще, н≥ж Sr—2O4.
” сильних м≥неральних кислотах, за вин€тком H2SO4, оксалати Ѕар≥ю, —тронц≥ю, альц≥ю розчин€ютьс€ добре.
ƒосл≥д 2. Ќайб≥льш достов≥рною реакц≥Їю ви€вленн€ кат≥он≥в —a2+ Ї м≥крокристалоскоп≥чна реакц≥€ утворенн€ кристал≥в кальц≥й сульфату.
ƒл€ проведенн€ ц≥Їњ реакц≥њ на предметне скло м≥кроскопа нанос€ть одну краплю розчину сол≥ альц≥ю, додають до ц≥Їњ крапл≥ краплю розчину сульфатноњ кислоти (с(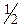 H2SO4)=2,0 моль/дм3). ”творюютьс€ кристали кальц≥й сульфату. ѓх р≥вном≥рно розпод≥л€ють скл€ною паличкою по склу так, щоб не утворювалось товстого шару кристал≥в, ≥ розгл€дають п≥д м≥кроскопом голчаст≥ кристали у вигл€д≥ пучк≥в або з≥рочок.
H2SO4)=2,0 моль/дм3). ”творюютьс€ кристали кальц≥й сульфату. ѓх р≥вном≥рно розпод≥л€ють скл€ною паличкою по склу так, щоб не утворювалось товстого шару кристал≥в, ≥ розгл€дають п≥д м≥кроскопом голчаст≥ кристали у вигл€д≥ пучк≥в або з≥рочок.
–ис. Е ристали CaSO4Ј2H2O
«а на€вност≥ у досл≥джуваному розчин≥ вс≥х кат≥он≥в третьоњ анал≥тичноњ групи до розчину додають розчин сульфатноњ кислоти (с(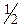 H2SO4)=2,0 моль/дм3), нагр≥вають, пот≥м центрифугують. ¬ осад≥ будуть BaSO4 та SrSO4 ≥ т≥льки частково —аSO4. раплю центрифуга- ту пом≥щають на предметне скло м≥кроскопа та упарюють до по€ви б≥лоњ кайми, а пот≥м розгл€дають утворен≥ кристали п≥д м≥кроскопом.
H2SO4)=2,0 моль/дм3), нагр≥вають, пот≥м центрифугують. ¬ осад≥ будуть BaSO4 та SrSO4 ≥ т≥льки частково —аSO4. раплю центрифуга- ту пом≥щають на предметне скло м≥кроскопа та упарюють до по€ви б≥лоњ кайми, а пот≥м розгл€дають утворен≥ кристали п≥д м≥кроскопом.
ƒосл≥д 3. ал≥й хромат K2CrO4 (CrO42Ц) з йонами —a2+ утворюЇ добре розчинний у вод≥ CаCrO4:
—a2+ + CrO42Ц → CаCrO4.
“ому при зм≥шуванн≥ розчин≥в в≥дпов≥дних солей у нейтральному середовищ≥ може зТ€витис€ лише легке помутн≥нн€, а в кислому середовищ≥ (нав≥ть оцтовокислому) н≥€ких ознак утворенн€ осаду не спостер≥гаЇтьс€.
ƒосл≥д 4. ат≥они —a2+ при взаЇмод≥њ з кал≥й гексац≥ано(≤≤) фератом у присутност≥ NH4+-≥он≥в при нагр≥ванн≥ утворюють б≥лий кристал≥чний осад подв≥йноњ сол≥:
—a2+ + [Fe(CN)6]4Ц + 2NH4+ → —a(NH4)2[Fe(CN)6]↓.
ƒл€ виконанн€ ц≥Їњ реакц≥њ до розчину, що м≥стить йони —a2+, додають дек≥лька крапель розчин≥в 4[Fe(CN)6] та NH4Cl, пот≥м нагр≥вають до кип≥нн€. „ерез дек≥лька хвилин випадаЇ осад кальц≥й-амон≥й гексац≥ано(≤≤) ферату.
¬ оцтов≥й кислот≥ цей осад не розчин€Їтьс€. ÷€ реакц≥€ недостатньо чутлива. р≥м того, кат≥они Ba2+ при њх висок≥й концентрац≥њ при взаЇмод≥њ з 4[Fe(CN)6] також утворюють аналог≥чний осад ¬a(NH4)2[Fe(CN)6]. ат≥они ≥нших анал≥тичних груп (за вин€тком першоњ) теж утворюють осади з цим реактивом. “ому ц€ реакц≥€ практичного застосуванн€ в анал≥тичн≥й практиц≥ не маЇ.
ƒосл≥д 5. ѕопередньо ви€вити йони —a2+ можна за реакц≥Їю забарвленн€ полумТ€ пальника. Ћетк≥ сол≥ альц≥ю забарвлюють безбарвне полумТ€ пальника у цегельно-червоний кол≥р.






