ƒо четвертоњ анал≥тичноњ групи в≥днос€ть кат≥они Al3+, Cr3+, Zn2+, As3+, Sn2+, AsV, SnIV.
јлюм≥н≥й ≥ ÷инк мають пост≥йну ступ≥нь окисненн€ у сполуках, а ≥нш≥ елементи Ц зм≥нну.
≈лектронна будова кат≥он≥в ц≥Їњ анал≥тичноњ групи така:
Al3+ − [Ne]; Cr3+ − [Ar]3d3; Zn2+ − [Ar]3d10; As3+ − [Ar]4s23d10; Sn2+ −
[Kr]5s24d10.
√руповим реагентом на кат≥они четвертоњ анал≥тичноњ групи Ї надлишок NaOH чи ќЌ.
ѕри д≥њ луг≥в на кат≥они четвертоњ анал≥тичноњ групи утворюютьс€ в≥дпов≥дн≥ г≥дроксиди:
Al3+ + 3OHЦ → Al(OH)3↓; Zn2+ + 2OHЦ → Zn(OH)2↓;
Cr3+ + 3OHЦ → Cr(OH)3↓; Sn2+ + 2OHЦ → Sn(OH)2↓ ≥ ≥н.
√≥дроксиди јлюм≥н≥ю, ÷инку, —тануму б≥лого кольору, а хром(≤≤≤) г≥дроксид Ц с≥ро-зеленого забарвленн€.
”творен≥ г≥дроксиди мають амфотерн≥ властивост≥. ¬они легко розчин€ютьс€ в кислотах та лугах:
Al(OH)3 + 3H+ → Al3+ + 3Ќ2ќ;
Cr(OH)3 + 3H+ → Cr3+ + 3Ќ2ќ;
Zn(OH)2 + 2H+ → Zn2+ + 2Ќ2ќ;
Sn(OH)2 + 2H+ → Sn2+ + 2Ќ2ќ.
« лугами ус≥ кат≥они четвертоњ анал≥тичноњ групи (за вин€тком јрсену) утворюють розчинн≥ у вод≥ комплексн≥ йони:
Al(OH)3 + 3OHЦ → [Al(OH)6]3Ц;
Cr(OH)3 + 3OHЦ → [Cr(OH)6]3Ц;
Zn(OH)2 + 2OHЦ → [Zn(OH)4]2Ц;
Sn(OH)2 + 2OHЦ → [Sn(OH)4]2Ц.
¬трачаючи при нагр≥ванн≥ воду, комплексн≥ ан≥они розпадаютьс€ ≥ перетворюютьс€ в прост≥ ан≥они:
[Al(OH)6]3Ц → Alќ2Ц + 2Ќ2ќ + 2ќЌЦ;
алюм≥нат-≥он
[Cr(OH)6]3Ц → Crќ2Ц + 2Ќ2ќ + 2ќЌЦ;
хром≥т-≥он
[Zn(OH)4]2Ц → Znќ22Ц + 2Ќ2ќ;
цинкат-≥он
[Sn(OH)4]2Ц → Snќ22Ц + 2Ќ2ќ.
стан≥т-≥он
ислотн≥ властивост≥ цих ан≥он≥в зростають у такому напр€мку:
Crќ2− → Alќ2− → Znќ22Ц → Snќ22Ц → Asќ43Ц.
“аким чином, хром≥ти мають найб≥льш слабк≥, а арсен≥ти та арсенати Ц найб≥льш сильн≥ кислотн≥ властивост≥. « цього випливаЇ, що хром≥ти Ї досить нест≥йкими. ѕри кипТ€т≥нн≥ вони легко г≥дрол≥зуютьс€ з утворенн€м хром(≤≤≤) г≥дроксиду нав≥ть у слабко лужному середовищ≥:
Crќ2− + 2Ќ2ќ → Cr(OH)3↓ + OH−.
ислотн≥ властивост≥ Alќ2− дещо сильн≥ш≥, н≥ж Crќ2−. “ому алюм≥нати ст≥йк≥ш≥ хром≥т≥в ≥ при нагр≥ванн≥ повн≥стю г≥дрол≥зуютьс€ т≥льки у присутност≥ йон≥в амон≥ю:
Alќ2− + NH4+ + 2Ќ2ќ → Al(OH)3↓ + NH4OH.
÷инкати ≥ особливо стан≥ти, станати, арсен≥ти та арсенати б≥льш ст≥йк≥, н≥ж хром≥ти та алюм≥нати ≥ в лужних розчинах осад≥в не утворюють н≥ за €ких умов; цинкати, стан≥ти та станати г≥дрол≥зуютьс€, утворюючи осади г≥дроксид≥в лише з нейтральних розчин≥в, а арсен≥ти та арсенати осаду не утворюють нав≥ть при нейтрал≥зац≥њ розчину.
—л≥д зазначити, що Cr(OH)3 у присутност≥ кат≥он≥в Zn2+ не розчин€Їтьс€ у надлишку лугу внасл≥док утворенн€ нерозчинного хром цинкату Cr2(ZnO2)3 (або Cr2O3Ј3ZnO).
’ром(≤≤≤) г≥дроксид може сп≥восаджуватись разом з г≥дроксидами обальту, Ќ≥колу, а тому майже не розчин€ютьс€ у надлишку луг≥в у присутност≥ сполук цих елемент≥в.
Ќ≥ кат≥они Cr3+, н≥ кат≥они Zn2+ не утворюють осад≥в при д≥њ на них натр≥й ацетату. јле €кщо йони Cr3+ та Zn2+ присутн≥ у розчин≥ разом, то при њх взаЇмод≥њ з натр≥й ацетатом одразу випадаЇ осад, €кий майже нерозчинний у розчинах луг≥в. јналог≥чне спостер≥гаЇтьс€ коли у розчин≥ присутн≥ разом йони Cr3+ та Ni2+, Cr3+ та —о2+.
|
|
|
ќсади г≥дроксид≥в Al(OH)3, Fe(OH)3 та основних оцтовокислих солей AlOH(CH3COO)2, FeOH(CH3COO)2 наст≥льки сп≥восаджують Cr(OH)3, що хром(≤≤≤) г≥дроксид у њх присутност≥ також дуже погано розчин€Їтьс€ при д≥њ надлишку лугу.
÷е по€снюЇтьс€ тим, що йони Cr3+ осаджуютьс€ не у форм≥ хром(≤≤≤) г≥дроксиду, а у вигл€д≥ в≥дпов≥дних солей, €к≥ у надлишку лугу розчин€ютьс€ погано:
2Cr3+ + 3Zn2+ +12ќЌ− → Cr2(ZnO2)3↓ + 6H2O;
2Cr3+ + 3—о2+ +8ќЌ− → Cо3(CrO2)2↓ + 4H2O;
2Al(OH)3 + 2Cr(OH)3 → Al2O3ЈCr2O3↓ + 6H2O;
CH3COO− + H2O → CH3COOЌ + ќЌ− (г≥дрол≥з);
CH3COOЌ + 12ќЌ− + 2Cr3+ + 3Zn2+ → Cr2(ZnO2)3↓ + 6H2O + CH3COOЌ.
“ак≥ властивост≥ кат≥он≥в Cr3+ та Zn2+ при анал≥з≥ кат≥он≥в четвертоњ анал≥тичноњ групи можуть бути використан≥ дл€ попереднього припущенн€ про њх на€вн≥сть у досл≥джуван≥й сум≥ш≥. якщо при д≥њ на розчин, що м≥стить сум≥ш кат≥он≥в четвертоњ анал≥тичноњ групи, розведеним розчином лугу ви€витьс€, що отриманий спочатку осад у надлишку лугу не розчин€Їтьс€, то це Ї ознакою того, що у розчин≥ на€вн≥ ≥ кат≥они Cr3+, ≥ кат≥они Zn2+. якщо ж осад у надлишку розведеного розчину лугу розчин€Їтьс€, то це означаЇ, що один з цих кат≥он≥в у розчин≥ в≥дсутн≥й.
ƒ≥ючи на сум≥ш кат≥он≥в четвертоњ анал≥тичноњ групи надлишком розчину амон≥аку, кат≥они Cr3+, Al3+, Sn2+ при цьому перейдуть у осад у вигл€д≥ в≥дпов≥дних г≥дроксид≥в, а йони Zn2+, As3+ та AsV залишатьс€ у розчин≥ у вигл€д≥ [Zn(NH3)4]2+ та AsO33Ц ≥ AsO43Ц.
якщо при д≥њ на розчин, що м≥стить кат≥они четвертоњ анал≥тичноњ групи, надлишком розчину лугу утворений спочатку осад повн≥стю розчин€Їтьс€, а при додаванн≥ до цього розчину невеликоњ к≥лькост≥ солей ’рому осад у надлишку розчину лугу не розчин€Їтьс€, це Ї ознакою на€вност≥ у розчин≥ кат≥он≥в Zn2+; €кщо ж до досл≥джуваного розчину додати невелику к≥льк≥сть сол≥ ÷инку, то у даному раз≥ нерозчинн≥сть осаду у надлишку розчину лугу вказуЇ на на€вн≥сть у цьому розчин≥ кат≥он≥в Cr3+.
ѕри необх≥дност≥ видалити з розчину кат≥они Zn2+ до розчину сл≥д додати приблизно екв≥валентну к≥льк≥сть сол≥ ’рому та под≥€ти на цей розчин надлишком розведеного розчину лугу. ѕри цьому вс≥ кат≥они Zn2+ будуть практично сп≥восаджен≥ з Cr(OH)3.
–озчинн≥ карбонати Na2CO3, K2CO3, (NH4)2CO3 осаджують кат≥они Al3+, Cr3+, Sn2+ та SnIV у вигл€д≥ в≥дпов≥дних г≥дроксид≥в, кат≥они Zn2+ Ц у вигл€д≥ основних солей. As3+ та AsV осад≥в не утворюють.
2Zn2+ + CO32Ц + 2Ќ2ќ → Zn2(ќЌ)2CO3↓ + 2Ќ+;
2Al3+ + 3CO32Ц + 3Ќ2ќ → 2Al(OH)3↓ + 3CO2;
2Cr3+ + 3CO32Ц + 3Ќ2ќ → 2Cr(OH)3↓ + 3CO2;
Sn2+ + CO32Ц + Ќ2ќ → Sn(OH)2↓ + CO2;
Sn4+ + 2CO32Ц + 2Ќ2ќ → Sn(OH)4↓ + 2CO2.
ќсновн≥ сол≥ ÷инку розчин€ютьс€ у розчин≥ амон≥аку у присутност≥ NH4Cl.
ат≥они Pb2+ у розчинах луг≥в утворюють б≥лий осад плюмбум г≥дроксиду, €кий у надлишку лугу розчин€Їтьс€ з утворенн€м комплексноњ сол≥:
Pb2+ + 2ќЌ− → Pb(OH)2↓;
Pb(OH)2 + 2NaOH → Na2[Pb(OH)4] Ц у розчин≥.
ѕроте розчиненн€ Pb(OH)2 у надлишку лугу в≥дбуваЇтьс€ пов≥льн≥ше, н≥ж розчиненн€ в≥дпов≥дних г≥дроксид≥в Al(OH)3, Cr(OH)3, Zn(OH)2, Sn(OH)2.
|
|
|
¬иход€чи з таких властивостей кат≥она Pb2+, його в≥днос€ть також ≥ до четвертоњ анал≥тичноњ групи кат≥он≥в.
ат≥они Sb3+ у значн≥й м≥р≥ теж в≥днос€тьс€ до розчин≥в луг≥в €к ≥ кат≥они четвертоњ анал≥тичноњ групи:
Sb3+ + 3ќЌЦ → Sb(OH)3↓;
б≥лий осад
Sb(OH)3 + NaOH → NaSbO2 + 2H2O.
ћайже вс≥ сол≥ кат≥он≥в четвертоњ анал≥тичноњ групи г≥дрол≥зуютьс€. ” найб≥льш≥й м≥р≥ г≥дрол≥зуютьс€ сол≥ —тануму та јрсену. “ому SnIV ≥ AsV у форм≥ кат≥он≥в Sn4+, As5+ можуть знаходитись т≥льки в кислих розчинах (переважно у розчинах хлоридноњ кислоти). ” нейтральних розчинах —танум переходить в осад в≥дпов≥дних г≥дроксид≥в, а јрсен Ц у форму ан≥она AsO43Ц або AsO33Ц:
Sn2+ + 2H2O → Sn(OH)2↓ + 2H+;
As5+ + 4H2O → AsO43Ц + 8H+.
¬с≥ сол≥ ’рому, јлюм≥н≥ю та ÷инку також у певн≥й м≥р≥ г≥дрол≥зуютьс€: водн≥ розчини хлорид≥в цих елемент≥в до певноњ м≥ри Ї кислими, њх сол≥ карбонатноњ кислоти п≥д д≥Їю води утворюють в≥дпов≥дн≥ г≥дроксиди, а цинк карбонат при г≥дрол≥з≥ переходить в основну с≥ль:
Al2(CO3)3 +3H2O → 2Al(OH)3↓ + 3CO2↑;
2ZnCO3 + H2O → (ZnOH)2CO3 + CO2↑.
ѕ≥д д≥Їю розчину амон≥аку кат≥они Al3+, Cr3+, Zn2+, Sn2+ також утворюють осади в≥дпов≥дних г≥дроксид≥в. Zn(OH)2 у надлишку розчину амон≥аку легко розчин€Їтьс€ з утворенн€м комплексного цинк амон≥акату:
Zn(OH)2 + 4NH3ЈH2O → [Zn(NH3)4](OH)2 + 4H2O.
ѕри д≥њ на розчин, що м≥стить кат≥они Al3+, Cr3+, Zn2+ г≥дро- фосфат-≥онами утворюютьс€ осади в≥дпов≥дних солей фосфатноњ кислоти, а йони Sn2+ осаджуютьс€ у вигл€д≥ Sn(OH)2; As3+ та As5+ при цьому осаду не утворюють:
Cr3+ + 2HPO42Ц → CrPO4↓+ H2PO4−;
3Sn2+ + 4HPO42Ц → Sn3(PO4)2↓+ 2H2PO4Ц.
”творений Sn3(PO4)2 дал≥ повн≥стю г≥дрол≥зуЇтьс€:
Sn3(PO4)2 + 6H2O → 3Sn(OH)2 + 2H3PO4;
3Zn2+ + 4HPO42Ц → Zn3(PO4)2↓+ 2H2PO4−;
Al3+ + 2HPO42Ц → AlPO4↓+ H2PO4−.
”творен≥ осади легко розчин€ютьс€ в м≥неральних кислотах та розчинах луг≥в, а CrPO4 та AlPO4 розчин€ютьс€ нав≥ть у оцтов≥й кислот≥.
ѕри д≥њ на сум≥ш кат≥он≥в четвертоњ анал≥тичноњ групи розчинами кал≥й гексац≥фано(≤≤) ферату чи кал≥й гексац≥ано(≤≤≤) ферату утворюютьс€ осади цинк гексац≥ано(≤≤) ферату або гексац≥ано(≤≤≤) ферату:
3Zn2+ + 2 4[Fe(CN)6] → Zn3 2[Fe(CN)6]2↓+ 6K+;
3Zn2+ + 2 3[Fe(CN)6] → Zn3[Fe(CN)6]2↓+ 6K+;
та осад Sn(OH)2:
2Sn2+ + 4[Fe(CN)6] + 4H2O → 2Sn(OH)2↓ + H4[Fe(CN)6] + 4K+.
ќсади кал≥й-цинк гексац≥ано(≤≤) ферату та цинк гексац≥ано(≤≤≤) ферату у вод≥ та розведених кислотах практично нерозчинн≥, внасл≥док чого реакц≥њ кат≥он≥в Zn2+ з 4[Fe(CN)6] або 3[Fe(CN)6] дають можлив≥сть ви€вл€ти дуже мал≥ концентрац≥њ у розчин≥ йон≥в Zn2+.
–озчин амон≥й сульф≥ду осаджуЇ кат≥они Al3+ та Cr3+ наступним чином:
2Al3+ + 3(NH4)2S → Al2S3 + 6NH4+;
2Cr3+ + 3(NH4)2S → Cr2S3 + 6NH4+.
”творен≥ алюм≥н≥й сульф≥д та хром сульф≥д миттЇво повн≥стю г≥дрол≥зуютьс€:
Al2S3 + 6H2O  2Al(ќЌ)3↓ + 3H2S;
2Al(ќЌ)3↓ + 3H2S;
Cr2S3 + 6H2O  2Cr(ќЌ)3↓ + 3H2S.
2Cr(ќЌ)3↓ + 3H2S.
ат≥они Zn2+ ≥з г≥дроген сульф≥дом за на€вност≥ ацетат-≥он≥в утворюють осад ZnS б≥лого кольору, €кий легко розчин€Їтьс€ в м≥неральних кислотах:
Zn2+ + H2S + 2—Ќ3—ќќ− → ZnS↓ + 2—Ќ3—ќќH.
як вже зазначалос€, As3+ та As5+ вход€ть до складу ан≥он≥в AsO33Ц та AsO43Ц. ≤ т≥льки у сильнокислому середовищ≥ вони ≥снують у вигл€д≥ кат≥он≥в As3+ та As5+. ѕри нагр≥ванн≥ у розчин≥ хлоридноњ кислоти в≥дбуваютьс€ реакц≥њ:
AsO33Ц + 6Ќ+ → As3+ + 3H2O;
AsO43Ц + 8Ќ+ → As5+ + 4H2O.
ј дал≥:
2As3+ + 3H2S → As2S3↓+ 6Ќ+;
2As5+ + 5H2S → As2S3↓+ 2S↓ + 10Ќ+.
ќсад As2S3 маЇ оранжево-жовте забарвленн€.
јналог≥чно взаЇмод≥ють з H2S йони Sn2+ та SnIV. ” сильно кислому середовищ≥ —танум ≥з ступенем окисненн€ 4+ знаходитьс€ у форм≥ кат≥она Sn4+:
Sn2+ + H2S → SnS↓+ 2Ќ+;
|
|
|
Sn4+ + 2H2S → SnS2↓+ 4Ќ+.
ќсад SnS маЇ буре забарвленн€, а SnS2 Ц бурувато-жовте.
4.5.1. –еакц≥њ ви€вленн€ кат≥он≥в Al3+
ƒосл≥д 1. ѕри д≥њ на розчин, що м≥стить йони Al3+, розчином амон≥аку в осад випадаЇ алюм≥н≥й г≥дроксид:
AlCl3 + 3NH4OH → Al(OH)3↓+ 3NH4Cl.
јлюм≥н≥й г≥дроксид не розчин€Їтьс€ в розчинах солей амон≥ю. якщо јлюм≥н≥й знаходитьс€ у розчин≥ у форм≥ алюм≥нату, то дл€ осадженн€ його розчином амон≥аку необх≥дно перевести алюм≥нат AlO2− у форму Al3+ д≥Їю м≥неральноњ кислоти:
AlO2− + 4H+ → Al3+ + 2H2ќ.
ƒосл≥д 2. ” амон≥ачному середовищ≥ Al(OH)3 утворюЇ з ал≥зарином та де€кими його пох≥дними (наприклад, S-ал≥зарином) малорозчинний комплекс €скраво червоного кольору, €кий називаЇтьс€ ал≥зариновим лаком:


–еакц≥ю на кат≥они Al3+ з ал≥зарином краще проводити крапельним методом. Ќа смужку ф≥льтрувального паперу нанос€ть
1-2 крапл≥ розчину сол≥ јлюм≥н≥ю, а пот≥м тримають 1-2хв. над скл€нкою з концентрованим розчином амон≥аку (у вит€жн≥й шаф≥). ѕри цьому кат≥они Al3+ осаджуютьс€ амон≥аком у вигл€д≥ Al(OH)3. Ќа отриману вологу пл€му нанос€ть краплю спиртового розчину ал≥зарину ≥ знову тримають в парах амон≥аку, при цьому ал≥зарин забарвлюЇтьс€ у червонувато-ф≥олетовий кол≥р оск≥льки утворюЇтьс€ амон≥й ал≥заринат. ƒл€ руйнуванн€ маскуючого забарвленн€ обережно п≥дсушують пап≥рець над полумТ€м пальника, забарвленн€ стаЇ бл≥до-жовтим, а пл€ма ал≥заринового лаку набуваЇ рожево-червоного забарвленн€. Ќа€вн≥ у досл≥джуван≥й сум≥ш≥ кат≥они Cr3+, Zn2+, Sn2+, Fe3+ заважають ви€вленню Al3+ таким способом. “ому у такому раз≥ ви€вленн€ кат≥он≥в Al3+ провод€ть наступним чином: на смужку ф≥льтрувального паперу нанос€ть краплю розчину K4[Fe(CN)6], а пот≥м в центр пл€ми внос€ть краплю досл≥джуваного на кат≥они Al3+ розчину. ѕ≥сл€ цього отримують пл€му, €ка забарвлена в центральн≥й частин≥, та вод€нистого безбарвного к≥льц€. ќсад в центр≥ пл€ми м≥стить сполуки Zn, Sn ≥ частково Cr3+, а кат≥они Al3+ м≥ст€тьс€ в перифер≥йн≥й частин≥.
÷ю пл€му витримують над парами амон≥аку, €кий осаджуЇ кат≥они Al3+ у вигл€д≥ Al(OH)3. ѕот≥м краплю перекреслюють кап≥л€ром, у €кому Ї розчин ал≥зарину, знову обробл€ють парами амон≥аку та висушують, тримаючи пап≥рець високо над полумТ€м пальника. «а на€вност≥ кат≥он≥в Al3+ зовн≥шн€ частина к≥льц€ в тих м≥сц€х, €к≥ були перекреслен≥ ал≥зарином, забарвлюютьс€ у оранжево-червоний кол≥р.
–еакц≥€ Al3+ з ал≥зарином Ї дуже характерною, але не специф≥чною. …они Cr3+, Zn2+, Sn2+ з ал≥зарином також утворюють в≥дпов≥дн≥ лаки: Cr3+ Ц буруватого, Zn2+ Ц червонуватого,
Sn2+ Ц цегельного забарвленн€, а SnIV Ц бл≥дожовтого забарвленн€. ѕроте ц≥ кат≥они заважають ви€вленню йон≥в Al3+ лише у тому випадку, коли њх концентрац≥њ у дес€тки раз≥в перевищують концентрац≥ю йон≥в Al3+. “ому при ви€вленн≥ кат≥он≥в Al3+ ал≥зарином вс≥ ц≥ кат≥они при значн≥й њх концентрац≥њ мають бути видален≥ ≥з розчину.
–озчин не повинен м≥стити також б≥льш≥сть кат≥он≥в ≥ ≥нших анал≥тичних груп, оск≥льки вони з ал≥зарином теж утворюють нерозчинн≥ лаки, причому багато з цих лак≥в мають забарвленн€ дуже схоже ≥з забарвленн€м алюм≥н≥Ївого лаку ≥ випадають у осад у тих же концентрац≥€х, що ≥ кат≥они Al3+. “ак, наприклад, кат≥они Fe3+ дають темно-ф≥олетовий лак, Ba2+, Sr2+, Ca2+, Sb3+, Bi3+, Cu2+ Ц лаки малинового забарвленн€, Cd2+ Ц оранжево-жовтий лак. Ќе утворюють ал≥заринов≥ лаки т≥льки кат≥они першоњ анал≥тичноњ групи, Ni2+, Mn2+, Mg2+, Pb2+, Ag+, Hg2+.
ƒосл≥д 3. ѕри д≥њ на розчин, що м≥стить кат≥они Al3+, розчином алюм≥нону утворюЇтьс€ внутр≥шньокомплексна с≥ль, що маЇ червоне забарвленн€. јлюм≥нон €вл€Ї собою амон≥йну с≥ль ауринтрикарбоновоњ кислоти:
|
|
|

ƒл€ проведенн€ ц≥Їњ реакц≥њ в проб≥рку внос€ть 3-5 крапель
досл≥джуваного розчину, додають 2-3 крапл≥ розчину оцтовоњ кислоти (с( —Ќ3—ќќЌ)=2,0моль/дм3) та 4-5 крапель алюм≥нону (w=0,1%). ѕроб≥рку ≥з сум≥шшю нагр≥вають, перем≥шують, додають розчин амон≥аку до лужноњ реакц≥њ середовища, а пот≥м 3-4 крапл≥ розчину амон≥й карбонату (с(
—Ќ3—ќќЌ)=2,0моль/дм3) та 4-5 крапель алюм≥нону (w=0,1%). ѕроб≥рку ≥з сум≥шшю нагр≥вають, перем≥шують, додають розчин амон≥аку до лужноњ реакц≥њ середовища, а пот≥м 3-4 крапл≥ розчину амон≥й карбонату (с(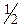 (NЌ4)2—ќ3=2,0моль/дм3). «а на€вност≥ кат≥он≥в Al3+ випадаЇ червоний осад, а €кщо кат≥он≥в Al3+ мало Ц червоне забарвленн€ розчину.
(NЌ4)2—ќ3=2,0моль/дм3). «а на€вност≥ кат≥он≥в Al3+ випадаЇ червоний осад, а €кщо кат≥он≥в Al3+ мало Ц червоне забарвленн€ розчину.
ƒосл≥д 4. ƒостатньо характерною реакц≥Їю на кат≥они Al3+ Ї реакц≥€ утворенн€ тенаровоњ син≥.
ƒл€ проведенн€ ц≥Їњ реакц≥њ у порцел€нову чашку внос€ть дек≥лька крапель досл≥джуваного на Al3+ розчину, 1-2 крапл≥ розведеноњ н≥тратноњ кислоти та дек≥лька крапель розведеного розчину кобальт н≥трату (с(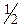 —о(Nќ3)2)=0,05моль/дм3). Ќа полумТњ пальника нагр≥вають чашку до повного випаровуванн€ р≥дини. «а на€вност≥ кат≥он≥в Al3+ утворюЇтьс€ синЇ забарвленн€ сухого залишку за рахунок кобальт алюм≥нату:
—о(Nќ3)2)=0,05моль/дм3). Ќа полумТњ пальника нагр≥вають чашку до повного випаровуванн€ р≥дини. «а на€вност≥ кат≥он≥в Al3+ утворюЇтьс€ синЇ забарвленн€ сухого залишку за рахунок кобальт алюм≥нату:
2Al2(SO4)3 + 2 —о(Nќ3)2 → 2Co(AlO2)2 + 6SO3↑ +4NO2↑ + O2↑.
синЇ забарвленн€
–обота проводитьс€ у вит€жн≥й шаф≥.
ѕроведенню ц≥Їњ реакц≥њ заважають кат≥они Zn2+, Cr3+, Cu2+, Ni2+, €к≥ з кобальт н≥тратом дають аналог≥чн≥ сполуки р≥зного забарвленн€.
ƒосл≥д 5. …они Al3+ можна ви€вити у слабкокислому середовищ≥ при рЌ≈4,5 за реакц≥Їю з 8-оксих≥нол≥ном. ѕри цьому утворюЇтьс€ комплекс алюм≥н≥й оксих≥нол≥нат, нерозчинний у вод≥ жовтого забарвленн€, €кий розчин€Їтьс€ у хлороформ≥.
¬и€вленню Al3+-≥он≥в за реакц≥Їю з оксих≥нол≥ном заважають Fe2+-≥они. ѓх окиснюють у Fe3+-≥они амон≥й персульфатом ≥ в≥дд≥л€ють екстракц≥Їю хлороформом при рЌ≈2,0, а алюм≥н≥й оксих≥нол≥нат залишаЇтьс€ при цьому у водн≥й фаз≥. «аважають ви€вленню Al3+-≥он≥в за цим методом йони Bi3+, Ni2+, Zn2+, Mg2+.

4.5.2. –еакц≥њ ви€вленн€ кат≥он≥в Cr3+
ƒосл≥д 1. ѕри взаЇмод≥њ кат≥он≥в Cr3+ з водним розчином амон≥аку утворюЇтьс€ осад хром(≤≤≤) г≥дроксиду с≥ро-зеленого забарвленн€:
Cr3+ + 3NH4OH → Cr(OH)3↓ + 3NH4+.
ƒосл≥д 2. ат≥они Cr3+ з розчинами луг≥в теж утворюють нерозчинний Cr(OH)3:
Cr3+ + 3OHЦ → Cr(OH)3↓.
јле в надлишку лугу Cr(OH)3 розчин€Їтьс€:
| Cr(OH)3 + OH− → [Cr(OH)4]− | 
| CrO2−. |
’ром≥т-≥они CrO2− мають €скраво-зелене забарвленн€.
Ќатр≥й хром≥т NaCrO2, на в≥дм≥ну в≥д натр≥й алюм≥нату NaAlO2,при нагр≥ванн≥ г≥дрол≥зуЇтьс€ з утворенн€м хром(≤≤≤) г≥дроксиду:
CrO2− + 2Ќ2ќ → Cr(OH)3↓ + OH−.
Ќатр≥й алюм≥нат при нагр≥ванн≥ залишаЇтьс€ у розчин≥ без зм≥н.
ƒосл≥д 3. ѕри д≥њ луг≥в на розчин, що м≥стить йони Cr3+, у присутност≥ кат≥он≥в Zn2+ утворюЇтьс€ осад хром цинкату, €кий нерозчинний у надлишку лугу:
2CrCl3 + 3ZnCl2 + 12NaOH → Cr2(ZnO2)3↓ + 12NaCl + 6H2O.
ƒосл≥д 4. ’арактерними реакц≥€ми на кат≥они Cr3+ Ї реакц≥њ окисненн€ цих йон≥в у лужному та кислому середовищах.
ќкисненн€ в лужному середовищ≥ можна проводити г≥дроген пероксидом чи натр≥й пероксидом Na2O2:
Cr3+ + 3OH− → Cr(OH)3↓;
Cr(OH)3 + OH− → [Cr(OH)4]− → CrO2− + 2H2O;
2CrO2− + 3Ќ2ќ2 + 2OH− → 2CrO42Ц + 4H2O.
ƒл€ проведенн€ ц≥Їњ реакц≥њ у проб≥рку внос€ть 3-4 крапл≥ розчину сол≥ хрому(≤≤≤), додають 2-3 крапл≥ розчину Ќ2ќ2 та 4 крапл≥ розчину NaOH. ¬м≥ст проб≥рки нагр≥вають прот€гом 3-4хв. «а рахунок утворених хромат-≥он≥в CrO42Ц розчин над осадом набуде жовтого забарвленн€. ѕ≥сл€ охолодженн€ осад в≥дф≥льтровують ≥ до ф≥льтрату додають розчин н≥тратноњ кислоти до по€ви оранжевого забарвленн€. ѕот≥м додають 6-8 крапель сум≥ш≥ д≥етилового етеру з ≥зоам≥ловим спиртом та 2-3 крапл≥ розчину Ќ2ќ2. —ум≥ш перем≥шують. ѕо€ва ≥нтенсивно синього забарвленн€ орган≥чноњ фази вказуЇ на утворенн€ надхромовоњ кислоти H2CrO6 (CrO5).
—л≥д мати на уваз≥, що окисненн€ Cr3+ до CrO42Ц маЇ в≥дбуватис€ у лужному середовищ≥, а перетворенн€ CrO42Ц в H2CrO6 Ц у кислому середовищ≥.
¬и€вленн€ кат≥он≥в Cr3+ за даною реакц≥Їю можна проводити у присутност≥ кат≥он≥в вс≥х анал≥тичних груп.
ќкисненн€ кат≥он≥в Cr3+ в кислому середовищ≥ кал≥й перманганатом KMnO4 в≥дбуваЇтьс€ до ан≥она Cr2O72Ц:
10Cr3+ + 6MnO4− + 11H2O → 5Cr2O72Ц + 6Mn2+ + 22Ќ+.
«а умови надлишку перманганат-≥он≥в можлива њх взаЇмод≥€ з утворенн€м Mn2+ Ц реакц≥€ самоокисненн€-самов≥дновленн€:
2MnO42Ц + 3Mn2+ + 7H2O → 5MnO(OH)2↓ + 4H+.
”творений при цьому осад MnO(OH)2 маЇ коричневе забарвленн€. якщо перманганат не був у надлишку, то тод≥ ф≥олетове забарвленн€ сум≥ш≥ переходить в оранжеве за рахунок утворених йон≥в Cr2O72Ц.
ƒл€ виконанн€ ц≥Їњ реакц≥њ у проб≥рку внос€ть 4-5 крапель розчину сол≥ ’рому(≤≤≤), 3-4 крапл≥ сульфатноњ кислоти та 10 крапель розчину KMnO4 (с( KMnO4)=0,1моль/дм3). Ќагр≥вають дек≥лька хвилин. ѕри цьому ф≥олетове забарвленн€ переходить в оранжеве.
KMnO4)=0,1моль/дм3). Ќагр≥вають дек≥лька хвилин. ѕри цьому ф≥олетове забарвленн€ переходить в оранжеве.
|
|
|
ќкиснити кат≥они Cr3+ до дихромат-≥он≥в Cr2O72Ц можна також амон≥й персульфатом у кислому середовищ≥:
2Cr3+ + 3S2O82Ц + 7H2O → Cr2O72Ц + 6SO42Ц + 14Ќ+.
ƒану реакц≥ю провод€ть у сильнокислому середовищ≥ при
рЌ=1-2 та при нагр≥ванн≥. ѕри цьому йони-в≥дновники заважають переб≥гу ц≥Їњ реакц≥њ.
ƒл€ проведенн€ даноњ реакц≥њ у проб≥рку внос€ть 5-6 крапель розчину амон≥й персульфату (NH4)2S2O8, додають краплю розчину сульфатноњ кислоти (с(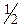 Ќ2SO4)=2,0моль/дм3) та краплю розчину AgNO3, €кий виступаЇ у рол≥ катал≥затора, а пот≥м 2-3 крапл≥ розчину хром(≤≤≤) сульфату чи хром(≤≤≤) н≥трату. ѕри цьому застосовувати €к джерело йон≥в Cr3+ хром хлорид не можна тому, що хлорид-≥они будуть окиснюватись за цих умов до в≥льного хлору, а з ≥ншого боку хлорид-≥они будуть звТ€зувати кат≥они Ag+ катал≥затора у нерозчинний осад AgCl. ѕ≥сл€ внесенн€ вс≥х компонент≥в проб≥рку нагр≥вають. ѕри цьому розчин набуваЇ оранжевого забарвленн€ внасл≥док утворенн€ ан≥он≥в Cr2O72Ц.
Ќ2SO4)=2,0моль/дм3) та краплю розчину AgNO3, €кий виступаЇ у рол≥ катал≥затора, а пот≥м 2-3 крапл≥ розчину хром(≤≤≤) сульфату чи хром(≤≤≤) н≥трату. ѕри цьому застосовувати €к джерело йон≥в Cr3+ хром хлорид не можна тому, що хлорид-≥они будуть окиснюватись за цих умов до в≥льного хлору, а з ≥ншого боку хлорид-≥они будуть звТ€зувати кат≥они Ag+ катал≥затора у нерозчинний осад AgCl. ѕ≥сл€ внесенн€ вс≥х компонент≥в проб≥рку нагр≥вають. ѕри цьому розчин набуваЇ оранжевого забарвленн€ внасл≥док утворенн€ ан≥он≥в Cr2O72Ц.
якщо цей розчин охолодити та додати до нього 2-3 крапл≥ розчину Ќ2ќ2, сум≥ш ≥зоам≥лового спирту та д≥етилового етеру ≥ збовтати, то верхн≥й орган≥чний шар забарвитьс€ в син≥й кол≥р внасл≥док утворенн€ хром пероксиду CrO5 або надхромовоњ кислоти H2CrO6:
Cr2O72Ц + 4Ќ2ќ2 + 2Ќ+ → 2H2CrO6 + 3H2O.
–еакц≥€ утворенн€ хром-пероксиду CrO5 надзвичайно чутлива, дозвол€Ї ви€вл€ти кат≥они Cr3+ та ан≥они CrO42Ц ≥ Cr2O72Ц у присутност≥ кат≥он≥в вс≥х анал≥тичних груп.
÷ю реакц≥ю сл≥д проводити виключно в кислому середовищ≥ при рЌ=2-3. Ќадхромова кислота H2CrO6 не ст≥йка у водному розчин≥, тому реакц≥ю провод€ть у сум≥ш≥ ≥зоам≥лового спирту та д≥етилового етеру, в €ких вона добре розчин€Їтьс€.
—туп≥нь окисненн€ ’рому €к у хром пероксид≥ CrO5, так ≥ в над- хромових кислотах становить 6+.

–едокс-потенц≥ал системи ≈ºCrO42Ц/CrO2Ц становить Ц0,12¬ (у лужному середовищ≥), а системи ≈ºCr2O72Ц/2Cr3+ Ц +1,36¬ (в кислому середовищ≥).
¬иход€чи з цього, окисниками кат≥он≥в Cr3+ у лужному середовищ≥ можуть бути хлорна чи бромна вода, амон≥й персульфат, кал≥й перманганат, Ќ2ќ2 та ≥нш≥ окисники, окисний потенц≥ал €ких у цьому середовищ≥ б≥льший за Ц0,12¬. ” кислому середовищ≥ кат≥они Cr3+ можна окиснити лише кал≥й перманганатом та амон≥й персульфатом, €к≥ мають окисний потенц≥ал б≥льший за +1,36¬ (≈ºMnO4−/Mn2+ = +1,52¬; ≈ºS2O82Ц/2SO42Ц = +2, 01 ¬).
ќкисненн€ кат≥он≥в Cr3+ до хром пероксиду або надхромових кислот в≥дбуваЇтьс€ т≥льки через дихромат-≥они та за участ≥ активного кисню:
Cr2O72Ц + 3ќ2Ц → 2CrO5,
або Cr2O72Ц + 5ќ2Ц → 2CrO62Ц.
ќтже, цей процес може в≥дбуватись лише в кислому середовищ≥, оск≥льки кисле середовище забезпечуЇ зм≥щенн€ р≥вноваги в б≥к утворенн€ дихромат-≥он≥в:
2CrO42Ц + 4Ќ+ → 2H2CrO4 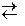 Cr2O72Ц + 2Ќ+ + Ќ2ќ.
Cr2O72Ц + 2Ќ+ + Ќ2ќ.
як окисник у цьому процес≥, зазвичай, використовують Ќ2ќ2:
Cr2O72Ц + 2Ќ2ќ2 → 2CrO5 + Ќ2ќ +2Ќ+
Cr2O72Ц + 3Ќ2ќ2 →2H2CrO6 + Ќ2ќ;
¬≥дома також надхромова кислота H3CrO8 червоного забарвленн€, у €к≥й ’ром маЇ ступ≥нь окисненн€ 5+:
 .
.
÷≥й кислот≥ в≥дпов≥даЇ хром пероксид складу Cr2O13:
 .
.
”творенн€ сол≥ надхромовоњ кислоти (H3CrO8) в≥дбуваЇтьс€ при окисненн≥ Cr3+ та в≥дновленн≥ CrO42Ц у лужному середовищ≥:
Cr3+ + 3ќЌ− → Cr(OH)3↓;
Cr(OH)3 + OH− → [Cr(OH)4]−;
[Cr(OH)4]− → CrO2− + 2H2O;
CrO2− + 5Ќ2ќ2 + 2OH− → CrO83Ц + 6H2O;
2CrO42Ц + 7Ќ2ќ2 + 2OH− → 2CrO83Ц+ 8H2O.
ѕри вибор≥ окисника дл€ окисненн€ йон≥в Cr3+ у кислому середовищ≥ з двох окисник≥в (KMnO4 або (NH4)2S2O8) сл≥д надати перевагу амон≥й персульфату, оск≥льки окисний потенц≥ал персульфат-≥она вищий, н≥ж перманганат-≥она, а також тому, що амон≥й персульфат безбарвний, що забезпечуЇ ч≥ткий перех≥д забарвленн€ системи ≥з зеленого до оранжевого.
ƒосл≥д 5. ¬и€вленн€ Cr3+-≥он≥в та CrO42Ц-≥он≥в за реакц≥Їю з дифен≥лкарбазидом
’ромат-≥они у слабкокислому середовищ≥ взаЇмод≥ють з дифен≥лкарбазидом, в≥дновлюючись при цьому до Cr3+, а дифен≥лкарбазид окиснюЇтьс€ до дифен≥лкарбазону. ƒифен≥лкарбазон з йонами Cr3+ утворюЇ забарвлений у червоно-ф≥олетовий кол≥р комплекс хром дифен≥лкарбазонату:


4.5.3. –еакц≥њ ви€вленн€ кат≥он≥в Zn2+
ƒосл≥д 1. ѕри д≥њ на розчин, що м≥стить кат≥они Zn2+, розчином амон≥аку спочатку утворюЇтьс€ осад б≥лого кольору цинк г≥дроксиду, €кий при додаванн≥ надлишку реагенту розчин€Їтьс€ з утворенн€м комплексного цинк амон≥акату:
Zn2+ + 2NH3ЈH2O → Zn(OH)2↓ + 2NH4+;
Zn(OH)2↓ + 4NH3ЈH2O → [Zn(NH3)4](OH)2 + 4H2O;
омплексний [Zn(NH3)4](OH)2 Ц безбарвний.
ƒосл≥д 2. « карбонатами лужних метал≥в та амон≥ю йони Zn2+ утворюють б≥лий осад основних солей:
2Zn2+ + 2—ќ32Ц + H2O → Zn2(OH)2—ќ3↓ + —ќ2↑.
ƒосл≥д 3. —ульф≥д-йони з кат≥онами Zn2+ утворюють б≥лий осад цинк сульф≥ду:
Zn2+ + Ќ2S → ZnS↓ + 2H+;
Zn2+ + S2Ц → ZnS↓.
ƒл€ виконанн€ ц≥Їњ реакц≥њ у проб≥рку внос€ть 3-4 крапл≥ розчину сол≥ ÷инку, додають 2-3 крапл≥ розчину натр≥й ацетату та 5-7 крапель розчину сульф≥дноњ кислоти чи розчини натр≥й, кал≥й чи амон≥й сульф≥д≥в. ¬изначають, при €кому значенн≥ рЌ розчину осад ZnS буде розчин€тис€. ƒана реакц≥€ в≥дбуваЇтьс€ краще в оцтовокислому середовищ≥. Ќа€вн≥сть у досл≥джуваному розчин≥ окисник≥в заважаЇ проведенню реакц≥њ оск≥льки сульф≥д-≥они Ї сильними в≥дновниками ≥ сам≥ легко окиснюютьс€.
ƒосл≥д 4. ал≥й гексац≥ано(≤≤) ферат K4[Fe(CN)6] з кат≥онами Zn2+ реагуЇ з утворенн€м б≥лого осаду подв≥йноњ сол≥:
3Zn2+ + 2[Fe(CN)6]4Ц + 2 + → 2Zn3[Fe(CN)6]2↓.
«а ц≥Їю реакц≥Їю можна в≥др≥знити кат≥они Zn2+ в≥д кат≥он≥в Al3+.
ƒосл≥д 5. ѕри д≥њ на розчин, що м≥стить йони Zn2+, хлороформним розчином д≥т≥зону (д≥фен≥лт≥окарбазону) утворюЇтьс€ хелатний комплекс червоного забарвленн€ Ц цинк д≥т≥зонат:

÷инк д≥т≥зонат забарвлюЇ у лужному середовищ≥ не т≥льки хлороформний шар, але ≥ верхн≥й водний, що дозвол€Ї ви€вл€ти йони Zn2+ у присутност≥ ≥нших кат≥он≥в.
ƒл€ проведенн€ ц≥Їњ реакц≥њ у проб≥рку внос€ть 2-3 крапл≥ досл≥джуваного на йони Zn2+ розчину, додають 5 крапель розчину натр≥й г≥дроксиду (с( NaOH)=2,0моль/дм3). якщо випадаЇ осад, його в≥дцентрифуговують. раплю центрифугату внос€ть у маленьку порцел€нову чашку та додають сюди 2-3 крапл≥ розчину д≥т≥зону в хлороформ≥, перем≥шують скл€ною паличкою до тих п≥р, доки весь хлороформ не випаруЇтьс€ (у вит€жн≥й шаф≥). «а на€вност≥ кат≥он≥в Zn2+ водний розчин набуваЇ рожевого або червоного забарвленн€. ѕри в≥дсутност≥ кат≥он≥в Zn2+ спостер≥гаЇтьс€ жовте забарвленн€ розчину натр≥й д≥т≥зонату.
NaOH)=2,0моль/дм3). якщо випадаЇ осад, його в≥дцентрифуговують. раплю центрифугату внос€ть у маленьку порцел€нову чашку та додають сюди 2-3 крапл≥ розчину д≥т≥зону в хлороформ≥, перем≥шують скл€ною паличкою до тих п≥р, доки весь хлороформ не випаруЇтьс€ (у вит€жн≥й шаф≥). «а на€вност≥ кат≥он≥в Zn2+ водний розчин набуваЇ рожевого або червоного забарвленн€. ѕри в≥дсутност≥ кат≥он≥в Zn2+ спостер≥гаЇтьс€ жовте забарвленн€ розчину натр≥й д≥т≥зонату.
якщо забарвленн€ спостер≥гаЇтьс€ т≥льки в шар≥ хлороформу, то це ще не Ї ознакою на€вност≥ йон≥в Zn2+.
ƒосл≥д 6. ат≥они Zn2+ можна усп≥шно ви€вити за певних умов м≥крокристалоскоп≥чною реакц≥Їю.
ƒл€ цього на предметне скло м≥кроскопа нанос€ть краплю досл≥джуваного розчину, п≥дкисненого оцтовою кислотою, додають краплю розчину амон≥й тетрародано(≤≤) меркурату (NH4)2[Hg(SCN)4]. «а на€вност≥ йон≥в Zn2+ утворюютьс€ кристали цинк тетрародано(≤≤) меркурату Zn[Hg(SCN)4]. ристали розгл€дають п≥д м≥кроскопом.
–ис. Е ристали цинк тетрародано(≤≤) меркурату
ристали з нейтральних чи лужних розчин≥в мають форму хрест≥в та дендрит≥в, а з п≥дкисненн€м розчин≥в Ц форми трикутник≥в або клин≥в. якщо досл≥джуван≥ розчини дуже розведен≥, то краплю такого розчину випарюють, а до сухого залишку додають краплю реагенту (NH4)2[Hg(SCN)4].
¬и€вленню кат≥он≥в Zn2+ за цим методом заважають кат≥они Cu2+, Ni2+, Cd2+ та ≥нш≥, а за на€вност≥ йон≥в —о3+ осад забарвлюЇтьс€ в син≥й кол≥р.
јмон≥й тетрародано(≤≤) меркурат (NH4)2[Hg(SCN)4] у присутност≥ йон≥в Cu2+ з кат≥онами Zn2+ у кислому середовищ≥ утворюЇ кристал≥чний оливково-зеленого кольору осад сум≥ш≥ купрум тетрародано(≤≤) меркурату та цинк тетрародано(≤≤) меркурату:
2(NH4)2[Hg(SCN)4] + Zn2+ + Cu2+ → —u[Hg(SCN)4]ЈZn[Hg(SCN)4] + 4NH4+.
«а на€вност≥ у розчин≥ йон≥в Zn2+ та —о2+ цей реактив утворюЇ ф≥олетово-блакитний осад цинк та кобальт тетрародано(≤≤) меркурат≥в:
2(NH4)2[Hg(SCN)4] + Zn2+ + Cо2+ → —о[Hg(SCN)4]ЈZn[Hg(SCN)4] + 4NH4+.
∆оден з кат≥он≥в четвертоњ анал≥тичноњ групи при будь-€к≥й њх концентрац≥њ не заважаЇ ви€вленню йон≥в Zn2+ за ц≥Їю реакц≥Їю.
ƒосл≥д 7. ат≥они Zn2+ можна ви€вити за реакц≥Їю утворенн€ р≥нмановоњ зелен≥. ÷ю реакц≥ю провод€ть наступним способом. —мужку ф≥льтрувального паперу просочують досл≥джуваним на кат≥они Zn2+ розчином та розчином Co(NO3)2, а пот≥м спалюють. «а на€вност≥ у розчин≥ кат≥он≥в Zn2+ поп≥л п≥сл€ спалюванн€ набуваЇ темно-зеленого забарвленн€, €ке обумовлене утворенн€м кобальт цинкату CoZnO2. ѕроведенню ц≥Їњ реакц≥њ заважають кат≥они Al3+, €к≥ у под≥бн≥й реакц≥њ утворюють алюм≥н≥й цинкат темно-синього кольору, та кат≥они Cr3+, Cu2+, Ni2+, €к≥ маскують своњм забарвленн€м кол≥р попелу.
ƒосл≥д 8. « ал≥зарином кат≥они Zn2+ утворюють лак червонуватого забарвленн€. јле ви€вити кат≥они Zn2+ за реакц≥Їю з ал≥зарином у присутност≥ ≥нших кат≥он≥в четвертоњ анал≥тичноњ групи (за вин€тком As3+ та AsV) неможливо, оск≥льки вс≥ вони утворюють з ал≥зарином лаки червонуватого чи жовтуватого забарвленн€.
4.5.4. –еакц≥њ ви€вленн€ кат≥он≥в Sn2+
—танум у сполуках може бути з≥ ступен€ми окисненн€ 2+ та 4+.
ƒосл≥д 1. ат≥он Sn2+ Ї сильним в≥дновником: ≈ºSn2+/Sn4+= +0,2¬. ÷€ властив≥сть використовуютьс€ дл€ ви€вленн€ цих йон≥в. …они Sn2+ в≥дновлюють кат≥они Bi3+ або Hg2+ до метал≥чних елемент≥в.
ƒл€ проведенн€ реакц≥й в≥дновленн€ кат≥он≥в Bi3+ та Hg2+ спочатку отримують стан≥т при взаЇмод≥њ йон≥в Sn2+ з лугом:
Sn2+ + 2ќЌ− → Sn(OH)2↓;
Sn(OH)2 + 2ќЌ− → [Sn(OH)4]2Ц;
[Sn(OH)4]2Ц → SnO22Ц + 2H2O.
ј дал≥: 2Bi3+ + 3SnO22Ц + 6ќЌ− → 2Bi↓ + 3SnO32Ц + 3H2O.
ƒл€ проведенн€ ц≥Їњ реакц≥њ у проб≥рку внос€ть 4 крапл≥ розчину, що м≥стить кат≥они Sn2+, додають розчин натр≥й г≥дроксиду (с( NaOH)=2,0моль/дм3) до розчиненн€ б≥лого осаду Sn(OH)2, а пот≥м додають 1-2 крапл≥ розчину сол≥ Ѕ≥смуту, наприклад Bi(NO3)2. «а на€вност≥ у досл≥джуваному розчин≥ йон≥в Sn2+ випадаЇ чорний осад метал≥чного б≥смуту.
NaOH)=2,0моль/дм3) до розчиненн€ б≥лого осаду Sn(OH)2, а пот≥м додають 1-2 крапл≥ розчину сол≥ Ѕ≥смуту, наприклад Bi(NO3)2. «а на€вност≥ у досл≥джуваному розчин≥ йон≥в Sn2+ випадаЇ чорний осад метал≥чного б≥смуту.
якщо в досл≥джуваному розчин≥ Ї йони Sb3+, то ви€вленн€ кат≥он≥в Sn2+ провод€ть у присутност≥ ан≥л≥ну. ƒл€ цього на смужку ф≥льтрувального паперу нанос€ть краплю розчину Bi(NO3)2, висушують, пот≥м нанос€ть краплю досл≥джуваного на йони Sn2+ розчину ≥ краплю ан≥л≥ну C6H5NH2.
«а на€вност≥ у розчин≥ кат≥он≥в Sn2+ через де€кий час на папер≥ зТ€вл€Їтьс€ темна пл€ма. ¬и€вленн€ йон≥в Sn2+ таким способом можна проводити у присутност≥ кат≥он≥в вс≥х анал≥тичних груп.
ƒосл≥д 2. ≤нша реакц≥€ ви€вленн€ кат≥он≥в Sn2+ Ц в≥дновленн€ у лужному середовищ≥ кат≥он≥в Hg2+ до в≥льноњ ртут≥:
Hg2+ + SnO22− + 2OH− → Hg↓ + SnO32Ц + H2O.
¬ипадаЇ чорний осад метал≥чноњ ртут≥.
ƒосл≥д 3. ат≥они Sn2+ у кислих розчинах можна ви€вити за реакц≥Їю з сульф≥дною кислотою:
Sn2+ + H2S → SnS↓ + 2H+.
”творюЇтьс€ темно-коричневий осад станум сульф≥ду SnS.
ƒосл≥д 4. ат≥они Sn2+ можна ви€вити в≥дновлюючи ними сполуки ћол≥бдену ≥з ступенем окисненн€ ћол≥бдену 6+. ѕри д≥њ на кислий розчин солей Sn2+ мол≥бденовою р≥диною (розчин (NH4)2MoO4 у розведених н≥тратн≥й або сульфатн≥й кислотах) або, ще краще, амон≥й фосформол≥бдатом ((NH4)3PO4Ј12MoO3) при високих концентрац≥€х реагенту та великому надлишку йон≥в Sn2+ 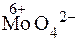 в≥дновлюЇтьс€ до
в≥дновлюЇтьс€ до  , що €вл€Ї собою с≥рий осад:
, що €вл€Ї собою с≥рий осад:
3Sn2+ + MoO42Ц + 4Ќ+ → 3Sn4+ + MoO2↓ + 2Ќ2ќ;
3Sn2+ + MoO3 + 2Ќ+ → 3Sn4+ + MoO2↓ + Ќ2ќ.
ѕри незначних концентрац≥€х йон≥в Sn2+ або мол≥бдат-≥он≥в, а також при надлишку MoO42Ц або MoO3 у пор≥вн€нн≥ з йонами Sn2+ розчин забарвлюЇтьс€ в ≥нтенсивно-син≥й кол≥р в результат≥ того, що йони Sn2+, окиснюючись до Sn4+, в≥дновлюють ћол≥бден ≥з ступенем окисненн€ 6+ до сполук, у €ких ћол≥бден маЇ ступ≥нь окисненн€ 5+. “ак≥ сполуки мають синЇ та зелене забарвленн€. ”творюЇтьс€ так звана Ђмол≥бденова синьї.
ќтримана таким способом Ђмол≥бденова синьї дуже нест≥йка. „ерез дек≥лька хвилин п≥сл€ њњ по€ви забарвленн€ розчину вже зникаЇ. якщо зам≥сть мол≥бденовоњ р≥дини застосовувати амон≥й фосформол≥бдат, то утворена Ђмол≥бденова синьї достатньо ст≥йка.
ƒану реакц≥ю з ви€вленн€ кат≥он≥в Sn2+ можна проводити крапельним методом. ƒл€ цього на смужку ф≥льтрувального паперу нанос€ть краплю розчину амон≥й фосформол≥бдату, а пот≥м на нењ Ц краплю досл≥джуваного розчину. «а на€вност≥ у розчин≥ йон≥в Sn2+ крапл€ набуваЇ синього забарвленн€.
÷€ реакц≥€ надзвичайно чутлива та специф≥чна на кат≥они Sn2+. “аким способом можна ви€вл€ти йони Sn2+ у присутност≥ не т≥льки кат≥он≥в четвертоњ анал≥тичноњ групи, але ≥ кат≥он≥в вс≥х ≥нших анал≥тичних груп.
ƒосл≥д 5. «а в≥дсутност≥ у досл≥джуваному розчин≥ кат≥он≥в Co2+, Ni2+, Cu2+, Cr3+, Mn2+, Sb3+ кат≥они Sn2+ дуже легко ви€вл€ютьс€ крапельним методом при взаЇмод≥њ њх з Fe3+ у присутност≥ диметилгл≥оксиму та винноњ кислоти чи натр≥й г≥дротартрату.
ƒл€ проведенн€ ц≥Їњ реакц≥њ на порцел€нову пластинку чи ф≥льтрувальний пап≥р нанос€ть 1-2 крапл≥ п≥дкисненого хлоридною кислотою досл≥джуваного розчину ≥ на цю краплю дал≥ нанос€ть
1-2 крапл≥ розчину FeCl3 та по одн≥й крапл≥ розчину винноњ кислоти чи NaHC4H4O6, розчину диметилгл≥оксиму та амон≥аку.
«а на€вност≥ у розчин≥ йон≥в Sn2+ зТ€вл€Їтьс€ €скраво-червоне забарвленн€, обумовлене тим, що утворен≥ у реакц≥њ йони Fe2+ з диметилгл≥оксимом та амон≥аком утворюють комплексну с≥ль, €ка маЇ червоне забарвленн€:
2Fe3+ + Sn2+ → 2Fe2+ + Sn4+

4.5.5. –еакц≥њ ви€вленн€ кат≥он≥в Sn4+
ќск≥льки зар€д кат≥он≥в у нейтральному розчин≥ не може бути б≥льшим, €к 3+, то кат≥он≥в Sn4+ у такому розчин≥ не ≥снуЇ. ” даному випадку сл≥д говорити про —танум у сполуц≥ з≥ ступенем окисненн€ 4+.
SnCl4 у водному розчин≥ сильно г≥дрол≥зуЇтьс€ тому €вл€Ї собою суспенз≥ю б≥лого кольору Sn(OH)4↓:
SnCl4 + 4HOH → Sn(OH)4 + 4HCl.
ѕри додаванн≥ до такоњ системи концентрованоњ хлоридноњ кислоти осад Sn(OH)4 розчин€Їтьс€ з утворенн€м комплексноњ сполуки, у €к≥й —танум з≥ ступенем окисненн€ 4+ знаходитьс€ у склад≥ комплексного ан≥она:
Sn(OH)4 + 6HCl → H2[SnCl6] + 4H2O.
” такому стан≥ —танум може бути в≥дновлений метал≥чним магн≥Їм чи метал≥чним зал≥зом до кат≥она Sn2+:
Mg + [SnCl6]2Ц → Mg2+ + Sn2+ + 6Cl−;
Fe + [SnCl6]2Ц → Fe2+ + Sn2+ + 6Cl−.
ƒл€ проведенн€ цих реакц≥й до досл≥джуваного розчину, що м≥стить —танум з≥ ступенем окисненн€ 4+, додають дек≥лька крапель концентрованоњ хлоридноњ кислоти, а пот≥м внос€ть ошурки метал≥чного магн≥ю чи метал≥чного зал≥за, нагр≥вають 3-4хв. ƒо отриманого розчину додають концентрований розчин лугу до сильно лужноњ реакц≥њ середовища. ѕри цьому Mg2+ включаЇтьс€ в осад Mg(OH)2, а Fe2+ Ц у Fe(OH)2, €кий дуже швидко окиснюЇтьс€ киснем пов≥тр€ до Fe(OH)3 бурого кольору. Sn2+ у надлишку лугу включаЇтьс€ в ан≥он SnO22Ц:
Sn2+ + 2OH− → Sn(OH)2↓;
Sn(OH)2 + 2OH− + [Sn(OH)4]2− → SnO22Ц + 2H2O.
” форм≥ стан≥ту SnO22Ц визначаЇтьс€ Sn2+ за реакц≥Їю в≥дновленн€ Bi3+ у метал≥чний б≥смут €к вже було розгл€нуто вище.
—танум з≥ ступенем окисненн€ 4+ можна також ви€вити д≥Їю на досл≥джуваний розчин з хлоридною кислотою розчином сульф≥дноњ кислоти:
H2[SnCl6] + 2H2S → SnS2↓ + 6HCl;
[SnCl6]2Ц + 2H2S → SnS2↓ + 4H+ + 6Clˉ
”творюЇтьс€ жовтий осад SnS2, €кий розчин€Їтьс€ у концентрован≥й хлоридн≥й кислот≥.
4.5.6. –еакц≥њ ви€вленн€ кат≥он≥в As3+ та As5+
ѕри робот≥ з≥ сполуками јрсену сл≥д не забувати, що вс≥ вони дуже токсичн≥!
як вже зазначалось, јрсен в досл≥джуваних розчинах знаходитьс€, зазвичай, у вигл€д≥ ан≥он≥в AsO33Ц або AsO43Ц. ” сильнокислому середовищ≥ арсен≥т-ан≥они частково переход€ть у кат≥он As3+:
AsO33Ц + 6Ќ+ → As3+ + 3H2O,
а арсенат-ан≥они частково переход€ть у кат≥он As5+:
AsO43Ц + 8Ќ+ → As5+ + 4H2O.
ƒосл≥д 1. ’арактерною реакц≥Їю кат≥он≥в As3+ та As5+ у сильнокислому середовищ≥ Ї њх взаЇмод≥€ з г≥дроген сульф≥дом Ќ2S:
2As3+ + 3H2S → As2S3↓ + 6H+,
2As5+ + 5H2S → As2S3↓ + 10H+ + 2S.
ќсадженн€ As2S3 краще в≥дбуваЇтьс€ з гар€чих розчин≥в сполук јрсену.
ƒл€ ви€вленн€ јрсену цим способом до 2-3см3 досл≥джуваного розчину додають 0,5см3 концентрованоњ хлоридноњ кислоти та нагр≥вають до 70-800—. ѕот≥м до нагр≥того розчину додають розчин сульф≥дноњ кислоти (г≥дрогенхлоридну воду). «а на€вност≥ у розчин≥ As3+ або As5+ випадаЇ осад As2S3 у вигл€д≥ жовто-коричневих крупинок. ¬и€вленню јрсену за ц≥Їю реакц≥Їю заважають кат≥они Sn2+, Sb3+, Bi3+, Cu2+, Hg2+, а також кат≥они Cd2+ при њх значн≥й концентрац≥њ у розчин≥.
ƒосл≥д 2. ѕри взаЇмод≥њ з магнез≥альною сум≥шшю (розчин MgCl2 у присутност≥ NH3ЈH2O та NH4Cl) йон≥в AsO43Ц утворюЇтьс€ б≥лий др≥бнокристал≥чний осад подв≥йноњ сол≥ NH4MgAsO4. …они AsO33Ц з магнез≥альною сум≥шшю осад не утворюють. ÷ю реакц≥ю можна проводити за в≥дсутност≥ ≥нших кат≥он≥в четвертоњ анал≥тичноњ групи, оск≥льки вс≥ вони з розчином амон≥аку, €кий входить до складу магнез≥альноњ сум≥ш≥, утворюють осади в≥дпов≥дних г≥дроксид≥в.
¬и€вленн€ јрсену за ц≥Їю реакц≥Їю провод€ть наступним способом. ƒо окремоњ проби досл≥джуваного розчину, що м≥стить кат≥они четвертоњ анал≥тичноњ групи, додають розчин Ќ2ќ2 або водний розчин йоду до слабкожовтого забарвленн€ дл€ окисненн€ йон≥в AsO33Ц до AsO43Ц, а пот≥м додають розчин NH3ЈH2O. ќтриманий при цьому осад г≥дроксид≥в Cr(OH)3, Al(OH)3, Sn2+ в≥дд≥л€ють центрифугуванн€м, а до центрифугату додають розчин NH4Cl дл€ попередженн€ утворенн€ осаду Mg(OH)2, а пот≥м краплинами внос€ть розчин MgCl2.
ат≥они Zn2+ при цьому або сп≥восаджуютьс€ з Cr(OH)3, або ж частково переход€ть у розчин у вигл€д≥ комплексноњ сол≥ [Zn(NH3)4]Cl2.
«а на€вност≥ у досл≥джуваному розчин≥ йон≥в AsO43Ц випадаЇ б≥лий др≥бнокристал≥чний осад NH4MgAsO4.
ƒосл≥д 3. …они AsO43Ц або As5+ у кислому середовищ≥ окиснюють йодид-≥они до в≥льного йоду, €кий можна ви€вити розчином крохмалю. –озчин забарвлюЇтьс€ у син≥й кол≥р:
AsO43Ц + 2I− + 2H+ → AsO33Ц + I2 + H2O;
As5+ + 2I− → As3+ + I2.
” нейтральному або слабколужному середовищ≥ йони As3+ або AsO33Ц в≥дновлюють в≥льний йод до йодид-≥она, тобто розчин йоду при цьому знебарвлюЇтьс€.
¬и€вл€ти јрсен за цими реакц≥€ми не можна у присутност≥ в розчин≥ кат≥он≥в Sn2+ або Sn4+, оск≥льки кат≥они Sn2+ також можуть в≥дновлювати в≥льний йод до йодид-≥он≥в, а кат≥они Sn4+ Ц окиснювати йодид-≥они до в≥льного йоду. ≤нш≥ кат≥они четвертоњ анал≥тичноњ групи не заважають ви€вленню јрсена за цими реакц≥€ми. « кат≥он≥в ≥нших анал≥тичних груп заважають переб≥гу цих реакц≥й кат≥они Fe3+, Fe2+, Sb3+, Cr3+, Cu2+, Ni2+, Co2+. ат≥они Fe2+ та Sb3+ реагують з йодом так, €к ≥ йони јрсену.
ƒосл≥д 4. « кат≥онами Ag+ у нейтральних розчинах арсенат-≥они утворюють шоколадно-бурий осад аргентум арсенату, а арсен≥т-≥они Ц жовтий осад аргентум арсен≥ту:
AsO43Ц + 3Ag+ → Ag3AsO4↓;
AsO33Ц + 3Ag+ → Ag3AsO3↓.
ќбидва ц≥ осади легко розчин€ютьс€ у розведен≥й н≥тратн≥й кислот≥ та в розчин≥ амон≥аку. ” розчин≥ амон≥аку утворюЇтьс€ комплексний кат≥он [Ag(NH3)2]+.
≤нш≥ кат≥они четвертоњ анал≥тичноњ групи не взаЇмод≥ють з
Ag+-≥онами за вин€тком SnIV. ¬насл≥док г≥дрол≥зу йона SnO32Ц утворюЇтьс€ при цьому б≥лий осад Sn(OH)4.
«а на€вност≥ у досл≥джуваному розчин≥ хлорид-≥он≥в при д≥њ на цей розчин розчином AgNO3 буде утворюватись осад AgCl, €кий нерозчинний у н≥тратн≥й кислот≥.
ƒосл≥д 5. ѕри д≥њ на арсенат-≥они мол≥бденовою р≥диною у присутност≥ Sn2+ розчин забарвлюЇтьс€ у син≥й кол≥р ≥ це забарвленн€ довго не зникаЇ. —л≥д зазначити, що под≥бне забарвленн€ з мол≥бденовою р≥диною виникаЇ з кат≥онами Sn2+ ≥ без AsO43Ц, але забарвленн€ при цьому швидко зникаЇ.
«а в≥дсутност≥ йон≥в Sn2+ арсенат-≥они з мол≥бденовою р≥диною у кислому середовищ≥ реагують з утворенн€м жовтого др≥бнокристал≥чного осаду:
AsO43Ц + 12(NH4)2MoO4 + 24H+ → (NH4)3AsO4Ј12MoO3↓ + 21NH4+ + 12H2O.
ѕри незначних концентрац≥€х реагуючих компонент≥в осад не утворюЇтьс€, а лише забарвлюЇтьс€ у жовтий кол≥р розчин. ÷ю реакц≥ю необх≥дно проводити при кипТ€т≥нн≥ прот€гом дек≥лькох хвилин.






