ѕример 1. ѕроизведение растворимости Ag2S при 298,15 составл€ет 6,90×10-50. ќпределить:
а) растворимость Ag2S в воде (моль/л, г/л);
б) растворимость Ag2S в 0,1 ћ растворе AgNO3;
в) растворимость Ag2S в 0,1 ћ растворе Na2S;
г) в каком количестве воды раствор€етс€ 1 г Ag2S;
д) DG0 процесса растворени€ и диссоциации Ag2S(к).
–ешение:
а) ѕусть растворимость Ag2S в воде составл€ет – моль/л. ¬ соответствии с уравнением растворени€ и диссоциации

концентрации ионов составл€ют: [S2Ц] = –, [Ag+] = 2P. ѕодставл€ем эти величины в выражение дл€ ѕ–:
ѕ– = [Ag+]2 [S2Ц] = (2P)2P = 4P3 = 6,90×10Ц50
и находим величину растворимости: – = 2,58×10Ц17 моль/л. ƒл€ нахождени€ растворимости в г/л полученное значение следует умножить на мол€рную массу Ag2S, получим 6,40×10Ц15 г/л.
б) “еперь Ag2S раствор€етс€ в растворе, где уже есть ионы серебра с концентрацией 0,1 моль/л. ѕусть растворимость Ag2S в этом случае составл€ет P1 моль/л. онцентрации ионов состав€т: [S2Ц] = P1; [Ag+] = 2P1 + 0,1.
ѕодставл€ем эти величины в выражение дл€ ѕP:
(2P1+0,1)2P1ї 0,01 P1 = 6,90×10-50.
Ќаходим растворимость Ag2S в 0,1 ћ растворе AgNO3:
P1 = 6,90×10Ц48 моль/л.
в) ¬ этом случае Ag2S раствор€етс€ в растворе, где уже есть анионы серы, их концентраци€ составл€ет 0,1 моль/л. ѕусть растворимость Ag2S в этом растворе составл€ет –2 моль/л; концентрации ионов состав€т: [S2Ц]=P2 +0,1; [Ag+] = 2P2.
ѕодставл€ем эти величины в выражение дл€ ѕ–:
ѕ– = (2P2)2 (P2+0,1)ї 0,4 P22 = 6,90×10Ц50.
Ќаходим растворимость Ag2S в 0,1 ћ растворе Na2S:
–2 = 4,15×10Ц25 моль/л.
ак видно, в растворах с общим ионом растворимость Ag2S меньше, чем в воде Ц наиболее резко упала растворимость в случае раствора AgNO3.
г) ¬ пункте а) была найдена растворимость Ag2S в воде в г/л, она составила 6,40×10Ц15. Ёта величина позвол€ет определить, в каком объеме воды раствор€етс€ 1 г Ag2S:
6,40×10Ц15 г Ц в 1л
1 г ††††††† †Ц в ’ л; †††††††††††††††††††††† откуда ’ = 1,56×1014 л.
д) ѕоскольку величина ѕ– представл€ет собой константу равновеси€, то значение DG0 процесса растворени€ и диссоциации Ag2S легко вычисл€етс€ по известному соотношению:
DG0 = Ц RTln ѕ– = Ц8,31×298,15ln 6,90×10Ц50 = 280,5 кƒж.
ѕример 2. ѕо справочным данным определить значение растворимости —аF2 при 298,15 .
–ешение. ѕервоначально находим значение ѕ– дл€ CaF2 по величине DG0 процесса:

DG0 = DG0обр—а2+(р-р, ст.с) + 2DG0обрFЦ (р-р, ст.с) Ц DG0обр—аF2(к) =
= Ц552,8 + 2(Ц277,7) Ц(Ц1168,5) = 60,3 кƒж.
DG0 = Ц RTlnѕ– = Ц8,31×298,15 ln ѕ–

ƒалее в соответствии с написанным выше уравнением растворени€ и диссоциации CaF2 растворимость этого соединени€ численно равна концентрации иона кальци€, а концентраци€ фторидного иона в 2 раза больше растворимости:
|
|
|
[Ca2+] = P; [FЦ] = 2P; ѕ– = [Ca2+] [FЦ] = P(2P)2 = 4P3 = 2,69×10Ц11.
ќтсюда растворимость равна:

ѕример 3. 0,1 г BaSO4 промыты 500 мл воды. ќпределить возможный процент потерь осадка вследствие растворимости, счита€, что промывные воды насыщены сульфатом бари€. ѕ– BaSO4 = 1,95×10Ц10.
–ешение. —огласно уравнению:

ѕ–BaSO4 = [Ba2+][SO42Ц] = P×P = P2,
где – Ц растворимость BaSO4 в воде.
Ќаходим –:
 моль/л или
моль/л или
1,39×10Ц5 моль/л × 233,4 г/моль = 3,26×10Ц3 г/л
—оответственно, в 500 мл насыщенного раствора будет находитьс€ 1,63×10Ц3 г BaSO4. ќтсюда возможна€ потер€ соли вследствиерастворимости осадка составит
 .
.
ѕример 4. —мешали 1 л 0,01 ћ раствора Pb(NO3)2 и « л 0,1 ћ раствора I. ¬ыпадет ли осадок –bI2, если ѕ–PbJ2 = «,56×10Ц9. —читать объем окончательного раствора равным 4 л.
–ешение. ƒл€ ответа на вопрос задачи следует вычислить произведение концентраций ионов –b2+ и IЦ в гипотетическом растворе после смешени€
и сравнить его с величиной ѕ–. ¬ исходных растворах концентрации ионов составл€ли соответственно:
[Pb2+] = 0,01; [IЦ] = 0,1 моль/л.
ѕосле смешени€ они состав€т:
 моль/л;
моль/л;
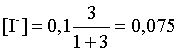 моль/л.
моль/л.
ѕроизведение концентраций ионов в полученном растворе составит
ѕ = [Pb2+][IЦ]2 = (0,0025)×(0,075)2=1,41×10Ц5.
ак видно, ѕ >ѕ–, следовательно осадок выпадет.
ѕример 5. ћассова€ дол€ растворенного вещества в насыщенном при 298,15 водном растворе CdS составл€ет 1,19×10Ц15. ќпределить ѕ– этого соединени€, полага€, что плотность раствора равна 1 г/мл.
–ешение. ¬ 1 кг раствора (1 л раствора) находитс€ 1000×1,19×10Ц15 = = 1,19×10Ц12 г CdS, что составл€ет 1,19×10Ц12: MCdS = 8,26×10Ц15 моль.
—огласно уравнению растворени€ и диссоциации CdS:

концентрации ионов в насыщенном растворе состав€т: [Cd2+] = [S2Ц] = = 8,26×10Ц15 моль/л. Ќаходим значение ѕ–:
ѕ– = [Cd2+][S2Ц] = (8,26×10Ц15)2 = 6,82×10Ц29.






