–еагент Ї специф≥чним дл€ ≥он≥в натр≥ю. ¬ нейтральних або оцтовокислих розчинах в≥н утворюЇ з сол€ми натр≥ю кристал≥чний осад зеленкувато-жовтого кольору. ÷€ реакц≥€ виконуЇтьс€ краплинним способом. –≥вн€нн€ реакц≥њ можна записати так:
Na+ + Zn(UO2)3Ac8 + HAc = NaZn(UO2)3Ac9 + H+.
¬и€вленню ≥он≥в натр≥ю ц≥Їю реакц≥Їю не заважають ≥они K+, NH4+, Mg2+, Ba2+, Sr2+, Ca2+ (у 20-кратному надлишку), а також де€к≥ кат≥они третьоњ Ц пТ€тоњ груп. «аважають ≥они Hg22+, Sb3+, PO43−, AsO33−, AsO43−, €к≥ утворюють з реактивом б≥л≥ осади. ѕри додаванн≥ реагенту до розчину Fe3+ утворюЇтьс€ ацетатний комплекс зал≥за, тому розчин набуваЇ червоного кольору, але осад не випадаЇ, ≥ тому Fe3+ практично не заважаЇ ви€вленню натр≥ю.
якщо при д≥њ реагенту на досл≥джуваний розчин випадають б≥л≥ голчаст≥ кристали (це може статис€ при висок≥й концентрац≥њ ≥он≥в K+), до одержаного осаду додають дек≥лька краплин дистильованоњ води; цей осад розчин€Їтьс€, а осад NaZn(UO2)3Ac9 залишаЇтьс€.
«абарвленн€ полумТ€.
« кат≥он≥в першоњ групи лише сполуки кал≥ю та натр≥ю забарвлюють полумТ€ в характерн≥ кольори. ƒосл≥д виконують таким чином: вушко платиновоњ дротинки, впа€ноњ в скл€ну паличку, змочують концентрованою сол€ною кислотою ≥ прожарюють у безбарвному полумТњ газового пальника. ќперац≥ю обробки дротинки сол€ною кислотою повторюють, поки полумТ€ пальника не стане безбарвним. ќчищену дротинку вм≥щують у досл≥джуваний розчин (або суху досл≥джувану речовину), а пот≥м у полумТ€. «а на€вност≥ натр≥ю полумТ€ забарвлюЇтьс€ в жовтий кол≥р, а за на€вност≥ кал≥ю Ц в бл≥до-ф≥олетовий. ƒосл≥д буде усп≥шним, коли провести попереднЇ концентруванн€ K+ на платинов≥й дротинц≥, застосувавши багаторазове змочуванн€ њњ досл≥джуваним розчином з наступним обережним висушуванн€м.
якщо у розчин≥ Ї обидва кат≥они, ≥он натр≥ю заважаЇ ви€вленню кал≥ю, тому що забарвленн€ його полумТ€ б≥льш €скраве ≥ воно забиваЇ забарвленн€ сол≥ кал≥ю.
≈кзаменац≥йний б≥лет 2
ѕомилки при проведенн≥ х≥м≥чного анал≥зу. математична обробка результат≥в анал≥зу.
ѕри проведенн≥ х≥м≥чного анал≥зу допускаЇтьс€ помилка €ка в≥дображаЇтьс€ на результатах анал≥зу
«а характером помилки бувають
¬ипадков≥ ≥ систематичн≥. ѕромахи- груб≥ помилки.
¬ипадков≥ помилки не можуть бути усуненн≥, але њх вплив може бути врахований статистичною оц≥нкою результат≥в анал≥зу. ¬ипадков≥ помилки можуть бути звТ€зан≥ з недотриманн€м операц≥й, чистоти реактив≥в.. —истематичн≥ помилки повТ€зан≥ з недосконал≥стю прилад≥в, з неправильним вибором методики. —истематична помилка обумовлена причинами в≥домоњ природи або ж причинами, €к≥ можуть бути ви€вленн≥ при детальному розгл€д≥ методики. ѓм в≥дпов≥даЇ пон€тт€ Ђправильн≥сть методу анал≥зуї
¬иди систематичних помилок
1.ћетодичн≥ (усуванн≥ ≥ не усуванн≥)
2. ќперативн≥
3. ≤ндив≥дуальн≥
ѕри статистичн≥й обробц≥ анал≥зу сл≥д розр≥зн€ти помилки, похибки результату анал≥зу.
—еред похибок розр≥зн€ють абсолютн≥ та в≥дносн≥ похибки.
 ⌂m= m≥ст-mрозрах.
⌂m= m≥ст-mрозрах.
|
|
|
⌂х=х≥ст- хроз.
≈= (m≥ст- mроз/ m≥ст )* 100%
E=(⌂/ m≥ст)*100%
Dсер= 

ƒостатньо дл€ характеристики в≥дтворювальност≥ результат≥в, чим менше dсер тим б≥льш точн≥ше визначенн€ викривленн€ похибки. Ѕ≥льш точн≥ше Ї стандартне в≥дхиленн€  , що Ї к≥льк≥сною м≥рою в≥дтворювальност≥ результат≥в
, що Ї к≥льк≥сною м≥рою в≥дтворювальност≥ результат≥в

якщо при 100 визначень 3 т≥льки вийдуть за меж≥ виборки. “од≥ буде розраховуватись дисперс≥€, або середнЇ квадратичне в≥дхиленн€.


ƒжерела похибок х≥м≥чного анал≥зу. ѕри виконанн≥ будь €кого анал≥зу звичайно провод€ть р€д анал≥тичних операц≥й (наприклад зважуванн€, розчиненн€, осаджуванн€, ф≥льтруванн€, осушуванн€, титруванн€, фотометруванн€.) ¬с≥ ц≥ анал≥тичн≥ операц≥њ ≥ вим≥рюванн€ можуть супроводжуватис€ похибками. Ќаприклад промиванн€ осаду може призвести до частковоњ втрати його внасл≥док розчинност≥ г≥гроскоп≥чноњ речовин≥ речовини при зважуванн≥ поглинаЇ вологу з пов≥тр€, що веде до зб≥льшенн€ њх маси, посп≥шн≥сть у к≥нц≥ титруванн€ неминуче веде до пере титруванн€.. Ќеточне вим≥рюванн€ шкали приладу (наприклад бюретки) в≥дкликаЇ до похибки вим≥рюванн€.
2. ƒруга анал≥тична група кат≥он≥в. ƒ≥€ групового реагенту. –еакц≥њ ви€вленн€ та в≥дкритт€ кат≥он≥в Ag+, Pb2+, Hg2+.
ат≥они другоњ анал≥тичноњ групи утворен≥ х≥м≥чними елементами р≥зних груп пер≥одичноњ таблиц≥: першоњ (Ag), другоњ (Hg) груп поб≥чних п≥дгруп те четвертоњ групи головноњ п≥дгрупи (Pb).
ќск≥льки в атом≥ Ag Ї d -, а в атом≥ Hg Ї d - ≥ f Ц стисненн€, то атоми мають близьк≥, невелик≥ рад≥уси ≥ велик≥ енергетичн≥ характеристики, слабк≥ в≥дновники. ¬они утворюють кат≥они з такими електронними формулами:
47 Ag +... 4s24p64d10 80 Ќg22+... 5s25p65d106 s1
ƒл€ них характерна:
—тех≥ометрична валентн≥сть ≤ (Ag +) ≥ ≤≤ (Ќg22+ )
—туп≥нь окисненн€ + 1 (Ag+, Ќg22+ )
«ар€д йон≥в 1+ (Ag+) або (Ќg22+)
ќксиди:
Ag2ќ Ц темно Ц коричневий
Ag+ + ќЌ - = AgќЌ↓
2 AgќЌ → Ag2ќ + Ќ2ќ
Ќg2ќ Ц чорного кольору у в≥льному стан≥ не вид≥лений
Ќg2ќ = Ќg↓ + Ќgќ
√≥дроксиди:
Ќg2(ќЌ)2 = Ќg2ќ+ Ќ2ќ
ƒ≥€ групового реагенту
Ag+ + —≤- = Ag—≤↓ аморфний с≥рнистий осад б≥лого кольору
Ќg22+ + 2—≤- = Ќg2—≤2↓ порошкопод≥бний б≥лий осад
Pb2+ + 2—≤- = Pb—≤2 ↓ б≥лий кристал≥чний осад
”мови: розведена хлоридна кислота, стех≥ометрична к≥льк≥сть речовини Ќ—≤, понижена температура.
–еакц≥њ Ag+ - кат≥она
« солей Ag+ - кат≥она добре розчин€ютьс€ у вод≥:
AgNO3, AgF, AgCIO3, AgCIO4, AgMnO4
ћалорозчинн≥: AgCH3COO Ag2SO4 AgNO2
’арактерн≥ реакц≥њ Ag+
1. ’лоридна кислота Ќ—≤ та њњ сол≥ осаджують з нейтральних розчин≥в
Ag+ - кат≥он
AgNO3 + Ќ—≤ = Ag—≤↓ + ЌNO3
Ag+ + —≤- = јg—l
2. NaOH, або ќЌ з Ag утв. чорний осад Ag2ќ
јg—l + NaOH= Ag2ќ + H2O + NaCl
Ag+ +OH -→ Ag2ќ + H2O
3. NH3ЈH2O з Ag утв. чорний осад Ag2ќ
Ag+ + 2 NH3ЈH2O → Ag2ќ + 2 NH4+ + H2O
4. ≤ - з Ag утв. жовтй осад Ag≤
Ag+ + ≤-→ Ag≤
ќсад Ag≤ розчин€Їтьс€ лише у присутност≥ великого надлишку ≤.
5. 2Cr2O7 з Ag утв. цегл€но-червоний осад Ag2 CrO4
2Ag++ CrO42-→ Ag2 CrO4
ќсад Ag2 CrO4 розчин€Їтьс€ в н≥тратн≥й кислот≥ в амон≥аку ≥ не розчин€Їтьс€ ацетатн≥й кислот≥. –еакц≥њ заважають йони плюмбуму, бар≥ю, стронц≥ю, меркур≥ю, кобальту.
5. Ќ2Dz з Ag утв. осад ф≥олетового кольору Ag ЌDz

6.Ќ2SO4 з Ag утв. осад б≥лого кольору Ag2SO4
2.Ag++ SO42-→ Ag2 CrO4
|
|
|
”мови реакц≥њ досить велика концентрац≥€ Ag+ ≥ SO42 йон≥в.
7. Ќ—ќќЌ Ц формальдег≥д в≥дновлюЇ Ag+ до в≥льного ср≥бла.
”мови реакц≥њ
- јмон≥ачно-лужна реакц≥€ розчину
- нагр≥ванн€

8. Na2S2O3 з Ag утв. осад б≥лого кольору Ag2S2O3
2.Ag+ + S2O32- → Ag2S2O3
ќсад розчин€Їтьс€ в надлишку реактиву завд€ки проходженню процесу комплексоутворенн€
Ag2S2O3 + S2O32- → 2[Ag (S2O3)2]3-
ќсад легко розчин€Їтьс€ у надлишку амон≥аку.
Ag—≤ + 2NH3 = [Ag(NH3)2]CI
ѕри д≥њ ЌNO3 випадаЇ б ≥лий осад
[Ag(NH3)2]CI + 2 ЌNO3 = Ag—≤↓ + 2 NH4Nќ3
[Ag(NH3)2]+ + —≤- + 2Ќ+= Ag—≤↓ + 2 NH4 +
–еакц≥€ фармакопейна.
–еакц≥њ Ќg22+ - кат≥она
Ѕ≥льш≥сть сполук д≥меркур≥й ≤ Ц кат≥она малолорозчинн≥ у вод≥.
Ќе розчинн≥: Hg2CI2,Hg2I2
ћалорозчинн≥: Hg2SO4, Hg2CrO4
–озчинн≥: Hg2(NO3)2, Hg2(CIO3)2, Hg2(CIO4)2
’арактерн≥ реакц≥њ Ќg22+
1. NaOH або KOH з Ќg22+ - йоном утворюЇ чорний осад
Ќg22+ + 2ќЌ- = Ќg2ќ↓ + Ќ2ќ
2. ≤ Ц йодид кал≥ю з Ќg22+ - йоном утворюЇ зелений осад
Ќg22+ + 2≤- = Ќg2≤2↓
ѕри надлишку кал≥ю йодиду
Ќg2≤2 + 2≤-= [Ќg≤4] + Ќg
3..NH3ЈH2O з Ќg22+ утв. Ѕ≥лий осад димеркур≥й ам≥д моно оксид н≥трату ≥ чорний осад в≥льноњ ртут≥.
Ќg22+ + 2 NH3ЈH2O + NO2-→ [ Ќg2ONH2]NO3 +2 Ќg + 2 NH4+ + H2O
4. 2Cr2O4 з Ќg 2+2 утв. Ќа холод≥ бурий аморфний осад Ќg2 CrO4, €кий при кип€т≥н≥ переходить у червоний кристал≥чний осад.
Ќg 2+2 + CrO42-→ Ќg2 CrO4
ќсад не розчин€Їтьс€ в лугах ≥ н≥тратн≥й кислот≥
5. упрум в≥дновлюЇ Ќg 2+2 до в≥льноњ ртут≥
Ќg 2+2 + —u → —u2++ 2 Ќg
”мови досл≥ду: м≥дна пластинка обезжирена ≥ очищена наждачним папером, в≥дсутн≥сть сильних окисник≥в.
6. ƒифен≥лкарбазон ≥ дифен≥лкарбазит з Ќg 2+2 дають сполуку блакитного кольору або синьо ф≥олетового.
ƒифен≥л карбазон ≥ ƒифен≥лкарбазит


”тв з Ќg 2+2 сполуку
 димеркур≥й(≤) дифен≥л карбазонат.
димеркур≥й(≤) дифен≥л карбазонат.
”мови реакц≥њ: в≥дсутн≥сть Ќg (≤≤) Ц кат≥она ≥ CrO4Чан≥она в кислому розчинн≥.
¬≥дсутн≥сть —r(III), Fe(II), Fe(III), Co(II) i Cu(II) кат≥он≥в у нейтральному ≥ ацетатнокислому розчинах.
7. Ќ2SO4 з Ќg22+ утв. осад б≥лого кольору Ќg2SO4, €кий розчин€Їтьс€ у в концентрован≥й н≥тратн≥й кислот≥.
Ќg22+ + SO42-→ Ќg2SO4
8. Na2CO3 або K2CO3 з Ќg22+ утв. ∆овтуватий осад, нест≥йкий тому поступово с≥р≥Ї внасл≥док дисмутац≥њ.
Ќg22+ + —ќ32- -→ Ќg2—O3
Ќg2—O3→ ЌgO + Ќg + —O2
’арактерн≥ реакц≥њ на плюмбум(≤≤) кат≥он
1. NaOH або KOH з –b2+ - йоном утворюЇ б≥лий осад Pb(OH)2
–b2+ + 2ќЌ- = Pb(OH)2
Pb(OH)2 амфотерний розчин€Їтьс€ в NaOH або KOH, HNO3.
Pb(OH)2 + 2OH-→ [Pb(OH)4]2-
Pb(OH)2+ CH3COOH→ Pb(CH3COO)2 + H2O
2. ≤ Ц йодид кал≥ю з –b2+ - йоном утворюЇ жовтий осад
–b2+ + 2≤- = –b≤2↓
–b≤2 + —Ќ3—ќќЌ→ Pb(CH3COO)≤
”мови:—тех≥ометрична к≥льк≥сть речовини реактиву ≤
3.NH3ЈH2O з –b2+ утв. ѕлюмбум г≥дроксид €кий не розчин€Їтьс€ в надлишку реактиву.
–b2+ + 2 NH3ЈH2O→ Pb(OH)2+ 2 NH4+
4. 2Cr2O4 ≥ 2Cr2O7 з –b2+ утв. жовтий др≥бнокристал≥чний осад плюмбуму
2–b2+ + CrO42-→ –bCrO4
2–b2+ + Cr2O72-+ 2 —Ќ3—ќќ- +ЌќЌ→2–bCrO4 + 2—Ќ3—ќќЌ
–bCrO4 Ц розчин€Їтьс€ в лугах ≥ практично не розчин€Їтьс€ в ам≥аку, в амон≥й ацетат≥.
5. Ќ2SO4 з –b2 + утв. б≥лий кристал≥чний осад –bSO4,
–b2 + + SO42-→ –bSO4,
6. Na2CO3 або K2CO3 з –b2+ утв. –b2+ осад б≥лого кольору.
–b2++ CO32- → –bCO3
7. SCN з –b2+ утв б≥лий осад.
–b2 + + 2 SCN-→ –b(SCN)2
- ¬плив однойменних та сторонн≥х йон≥в на розчинн≥сть електрол≥т≥в. —ольовий ефект.
—ольовий ефект це п≥двищенн€ розчинност≥ малороз≠чинних електрол≥т≥в, €к≥ перебувають у р≥вноваз≥ з осадом, при додаванн≥ до них сильних електрол≥т≥в, що не м≥ст€ть одноймен≠них йон≥в з осадом.
Ќаприклад, експериментально встановлено, що роз≠чинн≥сть сульфат≥в —аS04, SrS04 ≥ ¬аS04 зб≥льшуЇтьс€ при дода≠ванн≥ до њх насичених розчин≥в, €к≥ перебувають у р≥вноваз≥ з оса≠дом, розчин≥в —1, Nа—≤, KNO3, розчинн≥сть цих суль≠фат≥в тим б≥льша, чим б≥льша концентрац≥€ речовини добавленоњ сол≥ у розчин≥.
|
|
|
—ольовий ефект по€снюЇтьс€ на основ≥ вченн€ про ак≠тивн≥сть йон≥в ≥ йонну силу розчину. ѕри доливанн≥ до насичено≠го розчину малорозчинного електрол≥ту ≥ншого, сильного елект≠рол≥ту, €кий не маЇ сп≥льних йон≥в з малорозчинним елект≠рол≥том, йонна сила розчину зростаЇ, а коеф≥ц≥Їнти активност≥ йон≥в зменшуютьс€. ÷е веде до того, що величина добутку роз≠чинност≥ малорозчинного електрол≥ту стаЇ б≥льшою, н≥ж величи≠на добутку розчинност≥ цього електрол≥ту в чист≥й вод≥.
«а теор≥Їю сильних електрол≥т≥в сольовий ефект обумов≠лений зменшенн€м коеф≥ц≥Їнт≥в активност≥ йон≥в внасл≥док п≥д≠вищенн€ йонноњ сили розчину при додаванн≥ сторонн≥х електро≠л≥т≥в.
–≥зн≥ електрол≥ти, прилит≥ в однаков≥й к≥лькост≥, дають р≥зний сольовий ефект, оск≥льки йони мають р≥зну величину за≠р€д≥в, що впливаЇ на величину йонноњ сили.
ќсадженн€ можна вважати практично повним, €кщо в роз≠чин≥ залишаЇтьс€ така к≥льк≥сть речовини осаджуваних йон≥в, €ка не заважаЇ в подальших операц≥€х розд≥ленн€ ≥ ви€вленн€ йон≥в. ќск≥льки константа ƒ– н≥коли не буваЇ р≥вною нулю, то осаджен≠н€ г≥е може, бути абсолютно повним. „астина осаджуваних йон≥в завжди залишаЇтьс€ у розчин≥. ƒл€ б≥льш повного осадженн€ до розчину додають де€кий надлишок реагенту - осаджувача.
ѕовнота осадженн€ йона залежить в≥д розчинност≥ сполу≠ки, у вигл€д≥ €коњ осаджуЇтьс€ цей йон. ѕрактично при осадженн≥ будь-€кого йона'даю ть п≥вторакратний надлишок осаджувача у пор≥вн€нн≥ з≥ьстех≥ометричним, коли осадженн€ буде повним. ѕроте дужо великий надлишок осаджувача веде до розчиненн€ осаду.(сольовий ефект).
¬плив однойменних йон≥в. якщо до насиченого розчину малорозчинного електрол≥ту долити розчин ≥ншого електрол≥ту, €кий м≥стить однойменний йон з осадом, то добуток мол€рноњконцентрац≥њ йон≥в стане б≥льшим за величину добутку розчин≠ност≥, розчин стаЇ пересиченим, а пересичен≥ розчини нест≥йк≥; при сто€нн≥ вони вид≥л€ють частину розчиненоњ речовини у ви≠гл€д≥ осаду. –озчинн≥сть малорозчинного електрол≥ту пони≠жуЇтьс€ в найб≥льш≥й м≥р≥ при введенн≥ в розчин того йона, стех≥ометричний коеф≥ц≥Їнт €кого у формул≥ вищий.
4. –озрахувати масовий вм≥ст Mg(ω, %) у сплав≥ з алюм≥н≥Їм, виход€чи з наступних даних: маса наважки сплаву Ц 1,0135г, грав≥метрична форма Ц Mg2P2O7, њњ маса Ц 0,1325г.
ƒано
 mн=1,0135г
mграв≥метричноњ форми=0,1325
Mg2P2O7 mн=1,0135г
mграв≥метричноњ форми=0,1325
Mg2P2O7
|
W-?
яка масова частка Mg у сплав≥-?
W( %)(Mg)= 
W( %)(Mg)=  * 100%=13%
* 100%=13%
¬≥дпов≥дь: ћасова частка магн≥ю становить 13%.
Ѕ≥лет є3
ѕитанн€ 1
√рав≥метричний (вагов.) метод анал≥зу Ц це метод к≥льк≥сного х≥м. анал≥зу, €кий ірунтуЇтьс€ на точному вим≥рюванн≥ маси речовини, що визначаЇтьс€ або њњ складових частин, що вид≥л€ютьс€ у чистому стан≥ або у вигл€д≥ в≥дпов≥дних сполук.
ќсновн≥ операц≥њ: попередн€ п≥дготовка скл€ного посуду; розрахунок наважки; вз€тт€ наважки; розчиненн€ анал≥зуючоњ речовини; осадженн€; ф≥льтруванн€; промиванн€ осаду; висушуванн€; прокалюванн€ ≥ зважуванн€; розрахунок результат≥в. ќсад, що утворюЇтьс€ при грав≥метричному анал≥з≥ повинен бути практично нерозчинним, в≥докремлюватис€ в≥д ф≥льтрату.
–озр≥зн€ють три вар≥анти ваговаго анал≥зу:
1) визначенн€ вологи Ц наважку р-ни висушують, волога випаровуЇтьс€, маса зменшуЇтьс€.
W(вологи)= 
2) визначенн€ золи Ц спалюють точну наважку р-ни, прокалюють залишок до тих п≥р поки маса перестане зменшуватис€:
W (золи) = 
3) по мас≥ осаду Ц речовину перевод€ть в осад, €кий ф≥льтрують,промивають, висушують, зважують ≥ по мас≥ визначають вм≥ст анал≥зуючоњ речовини.
W (¬а)= 
W (Fe)= 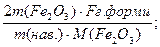
F Ц анал≥тичний фактор =  показуЇ масу в грамах визначуваного елемента в 1г осаду. F = 0,6994.
показуЇ масу в грамах визначуваного елемента в 1г осаду. F = 0,6994.
|
|
|
ѕитанн€ 2
ат≥они 3-оњ анал. гр утворюють х≥м. елементи 2 гр. головноњ п≥дгрупи пер≥од. системи, тобто лужноземельн≥ метали.
1) ƒ≥€ групового реактиву Ќ2SO4:
ће2+ + SO42- = ћеSO4¯, утв-с€ осади сульфат≥в Ц б≥л≥.
2) Na2CO3 Ц з кат≥онами 3-оњ анал≥т.гр. утворюЇ б≥л≥ аморфн≥ осади.
ће2+ + —ќ32- Ѓ ће—ќ3¯. ѕерев≥р€ють розчинн≥сть одержаних карбонат≥в y HCl, HNO3, CH3COOH. арбонати —а2+ - ¬а2+ легко розчин€ютьс€ у Ќ—l, HNO3, CH3COOH, ≥ H2CO3 у останньому випадку утворюютьс€ г≥дроген сол≥: CaCO3 + CO2 + H2OЃCa(HCO3)2.
3) (NH4)2CO3 Ц амон≥й карбонат з Ca2+, Sr2+, Ba2+ утворюЇ б≥л≥ аморфн≥ осади: ћеCl2+(NH4)2CO3ЃMeCO3¯+NH4Cl.
ƒ–(CaCO3)=1,7×10-8, SrCO3=9,4×10-10, BaCO3=4,9×10-9. “ому при кип€т≥нн≥ сульфат≥в CaSO4, BaSO4, SrSO4 з р-ном Na2CO3 в≥дбуваЇтьс€ перех≥д сульфат≥в в карбонати. MeSO4+CO32-Ѓ MeCO3¯+SO. BaCO3 розчин€ють у CH3COOH ≥ в≥дкривають ¬а2+характерними реакц≥€ми:
BaCO3+2CH3COOHЃ ¬а2+2CH3COO-+Ќ2ќ+—ќ2≠.
4) (NH4)2C2O4 Ц диамон≥й оксалат з —а2+ ≥ ¬а2+-≥онами утворюЇ б≥л≥ др≥бнокристал≥чн≥ осади оксалат≥в.:
ће2++—2ќ42-ЃMeC2O4¯. –озчинн≥сть оксалат≥в у CH3COOH р≥зна: —а—2ќ4 не розчин. в CH3COOH, Sr—2ќ4 дещо розчин€Їтьс€, ¬а—2ќ4 розчин€Їтьс€ в гар. CH3COOH.
5) Nа2Ќ–ќ4 з Ca2+, ¬а2+, Sr2+ утворюЇ б≥л≥ аморфн≥ осади —аЌ–ќ4, SrЌ–ќ4, ¬аЌ–ќ4.
ће2+ + Ќ–ќ42-ЃћеЌ–ќ4¯;
” присутност≥ амон≥аку утворюютьс€ середн≥ сол≥: —а3(–ќ4)2, Sr3(–ќ4)2, ¬а3(–ќ4)2.
3ће2++2Ќ–ќ42-+ NH3×Ќ2ќЃће3(–ќ4)2¯+ NH4+ + 2Ќ2ќ.
6) NаќЌ з Ca2+, ¬а2+, Sr2+ утворюЇ б≥л≥ осади, розчинн≥сть €ких у р€д≥ —а(ќЌ)2, Sr(ќЌ)2, ¬а(ќЌ)2 зростаЇ.
ће2+ + 2ќЌ-Ѓће(ќЌ)2¯.
6) (NH4)ќЌ не утворюЇ з цими кат≥онами осади.
’арактерн≥ та специф≥чн≥ реакц≥њ дл€ кат≥он≥в:
—а2+:
1) Nа2—6ќ6 Ц динатр≥й родизонат: ф≥олетове забарвленн€ у присут NаќЌ
CaCl2 + NаќЌ+ Nа2—6ќ6ЃCa(OH)2×CaC6O6 + NaCl.
2) K4[Fe(CN)6] - кал≥й гексац≥аноферат 2, утв-с€ кристал≥чний осад б≥лого кольору, склад €кого залежить в≥д умов:
Ca2+ + + + NH3×Ќ2ќ + [Fe(CN)6]4-Ѓ—аNЌ4K[Fe(CN)6]¯ + ќЌ-;
Ca2+2 NH4+ + [Fe(CN)6]4-Ѓ(NЌ4)2—а[Fe(CN)6]¯;
Ca2+ + 2 + +[Fe(CN)6]4-Ѓ 2—а[Fe(CN)6]¯;
2—а2+ + [Fe(CN)6]4-Ѓ —а2Fe(CN)6]¯;
3) ¬аF2 Ц утворюЇ б≥лий кристал осад —аF2:
Ca2+ + ¬аF2Ѓ —аF2+¬а2+.
4) 2CrO4- жовтий осад —аCrO4¯:
—а2+ + CrO42- + 2Ќ2ќЃ —аCrO4×2Ќ2ќ¯;
5) м≥крокристалоскоп≥чна р-ц≥€ —аSO4×2Ќ2ќ ви€вл€ють в присутност≥ Ќ2SO4;
6) р-ц≥€ на полумТ€ Ц цегл€но-червоне.
Sr2+:
1) Nа2—6ќ6 Ц утвор. Ѕурувато-червоний осад:
Sr2+ + —6ќ62-Ѓ Sr—6ќ6¯
2) р-ц≥€ з г≥псовою водою: (—аSO4×2Ќ2ќ)
Sr2+ + —аSO4Ѓ SrSO4¯+ —а2+, утв-с€ б≥лий кристал.осад.
3) 2CrO4- утв-с€ жовтий кристал осад:
Sr2+ + CrO42-Ѓ SrCrO4¯;
4) р-ц≥€ на полумТ€ Ц карм≥нно-червон.
¬а2+:
1) Nа2—6ќ6:
¬а2+ + —6ќ62-Ѓ¬а—6ќ6¯;
” присутност≥ Ќ+кат≥она переходить у г≥дроген-с≥ль:
¬а—6ќ6 + 2Ќ+ + —6ќ62-Ѓ¬а(Ќ—6ќ6)- червоно-бурого кольору
2) 2Cr2ќ7- з ¬а2+- кат≥оном утворюЇ жовтий осад бар≥й хромату(6):
2¬а2+ + Cr2ќ72-+ ЌќЌЃ2¬аCrќ4¯+2Ќ+
јбо
Cr2ќ72-+ ЌќЌЂCrќ42- + 2Ќ+;
2¬а2+ + 2 Crќ42-Ѓ2¬аCrќ4¯.
” водному розчин≥ Cr2ќ72- - ≥он г≥дрол≥зуЇ зутворенн€м Crќ42- - ≥она, €кий звТ€зуЇтьс€ ¬а2+- кат≥оном у осад. ¬елина ƒ–(¬аCrќ4) швидше дос€гаЇтьс€, н≥ж ƒ– (¬аCr2ќ7). ѕродуктом г≥дрол≥зу Ї Ќ+, €кий створюЇ кислу рекц≥ю розчину. ƒл€ усуненн€ заважаючоњ д≥њ Ќ+ додають —Ќ3—ќќNа.
3)конц. Ќ—l або HNO3 осаджують з досить концентрованих розчин≥в солей ¬а чист≥ сол≥.
4) K4[Fe(CN)6]- кал≥й гексац≥аноферат (2)з ¬а2+ утворюЇ б≥лий осад:
¬a2+ NH4+ + + + [Fe(CN)6]4-Ѓ¬а(NЌ4) [Fe(CN)6]¯;
5) р-ц≥€ на забарвленн€ полумТ€ Ц жовто-зелений кол≥р.
ѕитанн€ 3
ƒ»“»«ќЌ 
(дифен≥лт≥окарбазон, H2Dz) C2H5N=NC(S) NHNHC6H5,; чорн≥, пурпурно-чорн≥ або синьо-чорн≥ кристали; т. пл. 168∞«; не розч. у вод≥, дуже мало розч. у етанол≥, д≥етиловому еф≥р≥, розч. у —Ќ—l3 реагент дл€ екстракц≥йно-фотометрич. визначенн€ ≥ концентрац≥њ у вигл€д≥ однозам≥щених дитизонат≥в кат≥он≥в метал≥в, в т. ч. Ag(I), Au(III), Bi(III), Cu(I, II), Fe(II), Hg(I, II),
ƒиметилгл≥оксим  (р-ктив „угаЇва) застосовують дл€ €к≥сного ≥ к≥льк≥сного визначенн€ н≥келю. як селективний реагент на н≥кель диметилгл≥оксим запропонований в 1905 Ћ. ј. „угаевым, тому диметилгл≥оксим ≥нод≥ називають "реактив „угаева".
(р-ктив „угаЇва) застосовують дл€ €к≥сного ≥ к≥льк≥сного визначенн€ н≥келю. як селективний реагент на н≥кель диметилгл≥оксим запропонований в 1905 Ћ. ј. „угаевым, тому диметилгл≥оксим ≥нод≥ називають "реактив „угаева".
ѕри взаЇмод≥њ з ≥онами н≥келю диметилгл≥оксим утворюЇ червоний комплекс, €кий може бути легко обложений ≥ визначений грав≥метрично.
NiCl2 + NH3×Ќ2ќЃ[Ni(NH3)6]Cl2 + H2O
[Ni(NH3)6]Cl2 + H2Dm + 4H2OЃNi(HDm)2¯ + 2NH4Cl + 4NH3×Ќ2ќ
јл≥зарин (1,2-диг≥дроксиантрах≥нон)  - оранжево-червон≥ кристали(голки) трикл≥нноњ або ромб≥чноњ системи. ” анал≥тичн≥й х≥м≥њ ал≥зарин служить реагентом дл€ визначенн€ ≥он≥в алюм≥н≥ю ≥ р€ду ≥нших елемент≥в.
- оранжево-червон≥ кристали(голки) трикл≥нноњ або ромб≥чноњ системи. ” анал≥тичн≥й х≥м≥њ ал≥зарин служить реагентом дл€ визначенн€ ≥он≥в алюм≥н≥ю ≥ р€ду ≥нших елемент≥в.
|
|
|
Al3+ + H2Alis + 3OH-ЃAl(OH)2(HAlis) + H2O
8-оксих≥нол≥н (оксих≥нол≥н, оксин) - гетероцикл≥чна орган≥чна сполука складу C9H7NO. ясно-жовт≥ кристали; 
«датн≥сть 8-оксих≥нол≥на утворювати з багатьма кат≥онами метал≥в малорозчинн≥ у водних розчинах(оцтовоњ кислоти, ам≥аку та ≥н.) кристал≥чн≥ внутр≥шньокомплексн≥ сол≥(хелати), наприклад Mg(C9H6ON) 2, Al (C9H6ON) 3, використовуЇтьс€ на практиц≥ дл€ визначенн€ ≥ розд≥ленн€ р€ду метал≥в (Al, Zn, Cd, Mg та ≥н.).
1-н≥трозо-2-г≥дроксинафтал≥н з сол€ми кобальту(3)утворюЇ пурпурно-червоний осад, €кий не розчин€Їтьс€ в холодних HNO3, HCl, але розчин€Їтьс€ в орган. –озчинниках етанол,еф≥р.
2—оCl2 +  + 1/2ќ2Ѓ
+ 1/2ќ2Ѓ  +4HCl+H2O
+4HCl+H2O
ѕитанн€ 4-задача
ћ≈(—аCl2)=1/2*111г/моль=55,5г/моль;
m= ћ≈*C≈*V/1000=55,5*0,4*500/1000=11,1г;
“= m/V=11,1/1000=0,111г/см3.
Ѕ≥лет 4
3. ристал≥чний осад -це осад з пор≥вн€но сильними зв€зками м≥ж його частинками. ”творенн€ кристал≥чних осад≥в характеризуЇтьс€ тим, що при додаванн≥ окремих порц≥й осаджувача нов≥ центри кристал≥зац≥њ утворюютьс€ не в≥дразу. ќсновною умовою отриманн€ кристал≥чних осад≥в Ї пов≥льне додаванн€ реактиву, при €кому кристал≥чн≥ центри виростають у пор≥вн€но велик≥ кристали.
ƒл€ осадженн€ кристал≥чних осад≥в користуютьс€ розбавленими розчинами, так €к у цих умовах утворюютьс€ осади у форм≥ б≥льших кристал≥в. ѕри осаджуванн≥ кристал≥чних осад≥в з гар€чих розчин≥в зб≥льшуЇтьс€ њх розчинн≥сть, тому виникаЇ менше центр≥в кристал≥зац≥њ. ¬насл≥док цього створюютьс€ умови дл€ утворенн€ крупн≥ших кристал≥в.
ѕриклади: BaSO4, BaCrO4, CaC2O4 та ≥н.
јморфний осад характеризуЇтьс€ слабкими зв€зками м≥ж його частинками. ѕо сут≥ це коагульований внасл≥док швидкого доливанн€ розчину осаджувача колоњдний розчин. ѕри швидкому доливанн≥ реактиву Ї основною умовою отриманн€ аморфного осаду, в≥дразу виникаЇ багато центр≥в кристал≥зац≥њ та др≥бних агрегат≥в кристал≥в. јморфн≥ осади, особливо г≥дроксиди метал≥в, краще осаджувати з концентрованих розчин≥в. ÷е спри€Ї зменшенню загальноњ поверхн≥ ≥ утворенню осаду. ¬насл≥док зменшенн€ загальноњ поверхн≥ зменшуЇтьс€ адсорбц≥€ сторонн≥х речовин, що робить осади чист≥шими.
ѕри осаджуванн≥ аморфних осад≥в нагр≥ванн€ спри€Ї коагул€ц≥њ колоњдних частинок ≥ укрупненню зерен осаду.
јморфн≥ осади характерн≥ дл€ багатьох важкорозчинних сполук: г≥дроксид≥в та сульф≥т≥в метал≥в, сил≥катноњ та вольфрамовоњ кислот, та ≥нших.
ѕри осадженн≥ кристал≥чних осад≥в необх≥дно виконувати певн≥ умови:
1. осаджувати т≥льки з розведених розчин≥в розведеними розчинами осаджувача. ÷е упов≥льнюЇ випаденн€ осаду ≥ попереджаЇ забрудненн€ осаду
2. осаджувати лише п≥д≥гр≥т≥ розчини гар€чими розчинами осаджувач≥в, що також упов≥льнюЇ процес утворенн€ осаду ≥ робить його б≥льш крупнокристал≥чним
3. приливати осаджувач по крапл€м при пост≥йному перем≥шуванн≥ скл€ною паличкою, що зменшуЇ можлив≥сть адсорбц≥њ йон≥в осаджувача осадом.
ѕри осадженн≥ аморфних осад≥в необх≥дно виконуючи так≥ умови:
1. осаджувати т≥льки з концентрованих розчин≥в при нагр≥ванн≥
2. застосовувати осаджувач у вигл€д≥ концентрованого розчину ≥ додавати його швидко
3. добавл€ти п≥сл€ осадженн€ аморфного осаду 150 мл гар€чоњ води ≥ швидко ф≥льтрувати, щоб не в≥дбулас€ пептизац≥€ колоњда
”мови утворенн€ ≥ випад≥нн€ осад≥в п≥д час проведенн€ анал≥тичних реакц≥й: осад малорозчинного сильного електрол≥ту утворюЇтьс€ тод≥, коли п≥сл€ зм≥шуванн€ розчин≥в реагент≥в добуток мол€рних концентрац≥й кат≥он≥в ≥ ан≥он≥в (Iƒ) буде б≥льшим, н≥ж добуток розчинност≥ (ƒ–), тобто коли
Iƒ > ƒ–. Ќаприклад, осад бар≥й сульфату буде випадати тод≥, коли:
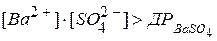 - розчин пересичений, переважаЇ процес
- розчин пересичений, переважаЇ процес
осадженн€, де  . якщо
. якщо  - динам≥чна р≥вновага; при
- динам≥чна р≥вновага; при  - розчин ненасичений, осад не випадатиме, а буде переважати процес розчиненн€ осаду.
- розчин ненасичений, осад не випадатиме, а буде переважати процес розчиненн€ осаду.
” загальному вигл€д≥ дл€ електрол≥ту  , €кий дисоц≥юЇ
, €кий дисоц≥юЇ

добуток розчинност≥ матиме вигл€д

 ,
,
де  ≥
≥  Ц стех≥ометричн≥ коеф≥ц≥Їнти в р≥вн€нн≥ дисоц≥ац≥њ електрол≥ту.
Ц стех≥ометричн≥ коеф≥ц≥Їнти в р≥вн€нн≥ дисоц≥ац≥њ електрол≥ту.
Ќаприклад, дл€ сол≥  , що дисоц≥юЇ, €к
, що дисоц≥юЇ, €к

добуток розчинност≥ буде таким
 .
.
1. “итруванн€ Ц це процес поступового добавл€нн€ розчину в≥домоњ концентрац≥њ з бюретки до розчину речовини, що визначаЇтьс€. –озчин, концентрац≥€ €кого в≥дома з високою точн≥стю, називаЇтьс€ титрованим, стандартним, робочим або титрантом. “итруванн€ ведуть до дос€гненн€ точки екв≥валентност≥ Ц це момент, коли речовини прореагують у в≥дпов≥дност≥ до закону екв≥валент≥в. “очку екв≥валентност≥ встановлюють за допомогою ≥ндикатор≥в. ≤ндикатор Ц це речовина, €ка за певних умов зм≥нюЇ св≥й кол≥р. ¬иб≥р ≥ндикатору залежить в≥д типу реакц≥њ.
¬имоги до реакц≥й в титриметричному анал≥з≥
–еакц≥њ в титриметричному анал≥з≥ повинн≥ задовольн€ти наступним вимогам:
1. –еакц≥њ повинн≥ проходити стех≥ометрично, тобто, проходити зг≥дно з р≥вн€нн€м реакц≥њ.
2. –еакц≥њ повинн≥ проходити к≥льк≥сно, тобто, повнота проходженн€ повинна складати не менше 99,9%.
3. —тандартний (титрований) розчин реактиву повинен реагувати т≥льки з речовиною, що визначаЇтьс€, тобто, реакц≥€ повинна бути специф≥чною.
4. –еакц≥€ м≥ж титрованим розчином ≥ розчином речовини, що визначаЇтьс€, повинна проходити з великою швидк≥стю.
5. Ќеобх≥дно мати над≥йний спос≥б ф≥ксуванн€ точки екв≥валентност≥.
6. ¬ розчин≥ повинн≥ бути в≥дсутн≥ми речовини, що заважають проходженню основноњ реакц≥њ або не дають можливост≥ ф≥ксувати точку екв≥валентност≥.
ласиф≥кац≥€ метод≥в титриметричного анал≥зу
¬ залежност≥ в≥д типу реакц≥њ, що лежить в основ≥ титруванн€, розр≥зн€ють наступн≥ методи анал≥зу.
1. ћетод кислотно Ц основного титруванн€
¬ основ≥ цього методу лежать кислотно Ц основн≥ реакц≥њ, повТ€зан≥ ≥з зм≥ною кислотност≥ розчину у процес≥ титруванн€. –≥зка зм≥на рЌ спостер≥гаЇтьс€ у точц≥ екв≥валентност≥. ƒл€ њњ ф≥ксац≥њ використовують рЌ-≥ндикатори, €к≥ зм≥нюють своЇ забарвленн€ в залежност≥ в≥д рЌ розчину.
2. ћетод окисно Ц в≥дновного титруванн€
¬ основ≥ цього методу лежать реакц≥њ окисненн€ Ц в≥дновленн€. ¬ процес≥ титруванн€ зм≥нюЇтьс€ потенц≥ал розчину. ƒл€ визначенн€ точки екв≥валентност≥ використовують окисно Ц в≥дновн≥ (редокс) ≥ндикатори, €к≥ зм≥нюють своЇ забарвленн€ в залежност≥ в≥д потенц≥алу розчину.
3. ћетод осадженн€ ≥ комплексоутворюванн€
“итриметричн≥ методи анал≥зу з використанн€м реакц≥й осадженн€ ≥ комплексоутворюванн€ обТЇднан≥ в одну групу метод≥в, оск≥льки багато реакц≥й осадженн€ за певних умов Ї ≥ реакц≥€ми комплексоутворюванн€, ≥ навпаки, реакц≥њ комплексоутворюванн€ зак≥нчуютьс€ утворенн€м малорозчинних сполук.
“очку екв≥валентност≥ в цих методах визначають €к ≥ндикаторними, так ≥ без ≥ндикаторними методами.
«а способом титруванн€ розр≥зн€ють методи пр€мого ≥ непр€мого титруванн€.
¬ методах пр€мого титруванн€ йон чи компонент, що визначаЇтьс€, титрують титрованим розчином або навпаки.
ƒо метод≥в непр€мого титруванн€ належать метод зам≥щенн€ та метод зворотного титруванн€ (метод залишк≥в).
ћетод зам≥щенн€ пол€гаЇ в тому, що йони, €к≥ визначаютьс€, зам≥щують екв≥валентною к≥льк≥стю ≥нших йон≥в, €к≥ вже можна визначити пр€мим титруванн€м. ÷ей метод використовують, €кщо Ї труднощ≥ з ф≥ксац≥Їю точки екв≥валентност≥.
ћетод зворотного титруванн€ (метод залишк≥в) пол€гаЇ в тому, що до анал≥зуЇмого розчину додають точно в≥домий надлишок титрованого розчину реактиву ≥ титрують цей надлишок в≥дпов≥дним робочим розчином. ћетод застосовують в тому випадку, коли немаЇ в≥дпов≥дного ≥ндикатора дл€ визначенн€ точки екв≥валентност≥, або, коли реакц≥€ проходить надто пов≥льно.
–озрахунки в титриметричному анал≥з≥
1. –озрахунок маси наважки, необх≥дноњ дл€ приготуванн€ заданого обТЇму стандартного розчину в≥домоњ концентрац≥њ
 , (5.6)
, (5.6)
де C-мол€рна концентрац≥€ екв≥валента стандартного розчину, моль-екв/л;  мол€рна маса екв≥валента речовини, г/моль-екв;
мол€рна маса екв≥валента речовини, г/моль-екв;  обТЇм розчину, мл.
обТЇм розчину, мл.
2. –озрахунки вм≥сту речовини за методом окремих наважок при пр€мому титруванн≥
 ; (5.7)
; (5.7)
 ; (5.8)
; (5.8)
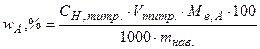 , (5.9)
, (5.9)
де  обТЇм титрованого розчину, мл;
обТЇм титрованого розчину, мл;  мол€рна концентрац≥€ екв≥валента титрованого розчину, моль-екв/л;
мол€рна концентрац≥€ екв≥валента титрованого розчину, моль-екв/л;  мол€рна маса екв≥валента речовини, що визначаЇтьс€, г/моль-екв;
мол€рна маса екв≥валента речовини, що визначаЇтьс€, г/моль-екв;  титр титрованого розчину ¬ за речовиною ј, г/мл;
титр титрованого розчину ¬ за речовиною ј, г/мл;  маса наважки, г.
маса наважки, г.
4. –озрахунки вм≥сту речовини при зворотному титруванн≥
 , (5.11)
, (5.11)
де  мол€рна концентрац≥€ екв≥валента титрованого розчину, надлишок €кого додаЇтьс€ до анал≥зуЇмого розчину, моль-екв/л;
мол€рна концентрац≥€ екв≥валента титрованого розчину, надлишок €кого додаЇтьс€ до анал≥зуЇмого розчину, моль-екв/л;  обТЇм надлишкового титрованого розчину, мл;
обТЇм надлишкового титрованого розчину, мл;  мол€рна концентрац≥€ екв≥валента титрованого розчину, €ким титруЇтьс€ надлишок, моль-екв/л;
мол€рна концентрац≥€ екв≥валента титрованого розчину, €ким титруЇтьс€ надлишок, моль-екв/л;  обТЇм цього титрованого розчину, мл;
обТЇм цього титрованого розчину, мл;  мол€рна маса екв≥валента речовини, що визначаЇтьс€, г/моль-екв;
мол€рна маса екв≥валента речовини, що визначаЇтьс€, г/моль-екв;  маса наважки, г.
маса наважки, г.
5. –озрахунки мол€рноњ маси екв≥валента
- кислоти 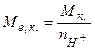 , (5.12)
, (5.12)
де  мол€рна маса кислоти, г/моль;
мол€рна маса кислоти, г/моль; 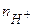 - число йон≥в Ќ+, що м≥стить кислота;
- число йон≥в Ќ+, що м≥стить кислота;
- основи  , (5.13)
, (5.13)
де 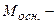 мол€рна маса основи, г/моль;
мол€рна маса основи, г/моль;  - число йон≥в ќЌ-, що м≥стить основа;
- число йон≥в ќЌ-, що м≥стить основа;
- сол≥  , (5.14)
, (5.14)
де  мол€рна маса сол≥, г/моль;
мол€рна маса сол≥, г/моль;  - к≥льк≥сть йон≥в металу;
- к≥льк≥сть йон≥в металу;  - зар€д йон≥в металу.
- зар€д йон≥в металу.
ƒл€ окисно-в≥дновних реакц≥й мол€рна маса екв≥валента речовини, що приймаЇ в н≥й участь, розраховуЇтьс€ за р≥вн€нн€м
 (5.15)
(5.15)
де  мол€рна маса речовини, г/моль;
мол€рна маса речовини, г/моль;  - к≥льк≥сть електрон≥в, що приймаЇ участь в окисно-в≥дновному процес≥ ц≥Їњ речовини.
- к≥льк≥сть електрон≥в, що приймаЇ участь в окисно-в≥дновному процес≥ ц≥Їњ речовини.
ќсновн≥ операц≥њ титриметричних метод≥в анал≥зу:
- приготуванн€ розчин≥в стандартноњ та анал≥зованоњ речовини.
- титруванн€
- обчисленн€
«а способом приготуванн€ розр≥зн€ють первинн≥ стандартн≥ розчини ≥ вторинн≥ стандартн≥ розчини- стандартизован≥.
—тандартний розчин Ц це розчин з точно в≥домою концентрац≥Їю речовини або точно в≥домим титром.
ѕервинн≥ стандартн≥ розчини (розчини з приготовленим титром): Na2B4O7 ∙ 10H2O, Na2CO3, K2Cr2O7, KCl, NaCl, Na2C2O4, H2C2O4 ∙ 2H2O.
—тандартизован≥ розчини: HCl, H2SO4, NaOH, KOH, KMnO4, Na2S2O3 ∙ 5H2O.
- –озвТ€зок
ƒопускають, що маса BaCl2 ∙ 2H2O = 0,1, оск≥льки осад кристал≥чний важчий за пов≥тр€
BaCl2 ∙ 2H2O - Ba
244 - 137,33
0,1 - x
X = 0,056 г
ω = m (практ. вих) / m (теор вих.) ω = 56, 77%
X = 0, 056 / 0, 5677 = 0, 099 г. (теор. вих.)
m (практ.в) = 0, 056 г.
m (теор. в) = 0, 099 г.
⌂ m (абсолютна) = │m (≥ст.) Ц m (розр.) │
⌂ m = │0, 099- 0, 056│
ε = │0, 099- 0, 056│ / 0, 056 ∙ 100% =76, 78 %
1. ƒ≥€ групового реактиву NaќЌ або ќЌ
Ќатр≥й г≥дроксид або кал≥й г≥дроксид з кат≥онами чет≠вертоњ анал≥тичноњ групи утворюють аморфн≥ осади г≥дроксид≥в, за вин€тком сполук As(III) ≥ јs(V):
[—г(OH2)6]3- + 3OH- → [Cr(OH2)3(OH)3]↓ + 3H2O
[Al(OH2)6]3+ + 3OH- → [Al(OH2)3(OH)3]↓ + 3H2O
[Zn(OH2)4]2+ + 2OH- → [Zn(OH2)2(OH)2]↓ + 2H2O
[SnCl4]2- + 2OH- → Sn(OH)2↓ + 4Cl-
Sn2+ + 2OH- → Sn(OH)2↓ або SnO ∙ xH2O
[SnCl6]2- + 4OH- → Sn(OH)4↓ + 6Cl-
Sn4+ + 4OH- → Sn(OH)4↓ або SnO2 ∙ xH2O
As3+ + 4OH- → [As(OH)4]-
As3+ + 6OH- → [As(OH)6]-
’ром(≤≤≤)-кат≥он
1. ќкисненн€ хром(≤≤≤)-кат≥она до —r(VI) р≥зними окисниками
а) ќкисненн€ хром(≤≤≤)-кат≥она г≥дроген пероксидом - Ќ2ќ2 у лужному середовищ≥:
2—r,3+ + 3Ќ2O2 + 10 ќH- → 2—rO42- + 8Ќ2O
2 | —r3+ + 8OЌ- - «е = —rO42- + 4Ќ2O
3 | H2O2 + 2e = 2OH-
б) ќкисненн€ хром(Ў)-кат≥она ћnO4 (кал≥й тетрaоксоманганатом(V≤≤)) у кислому середовищ≥:
10 —r3+ + 6ћnO4- + 11Ќ2ќ → 5—r2O72- + 6Mn2+ + 22Ќ+
5 | 2—r3+ + 7Ќ2O - 6е = —r2O72- + 14Ќ+
6 | ћnќ4- + 8Ќ+ + 5е = ћn2+ + 4Ќ2O
”мови реакц≥њ:
а) середовище сульфатноњ(”≤) кислоти;
в) ќкисненн€ хром(≤≤≤)-кат≥она (NH4)2S2O8 або 2S2O8 - диамон≥й або дикал≥й гексаоксопероксодисульфатом(V≤) у присутност≥ катал≥затора (AgNO3) ≥ н≥тратноњ(V) кислоти:
2—r3+ + 3S2O82- + 7Ќ2O → —r2O72- + 6SO42- + 14Ќ+
1 | 2Cr3+ + 7H2O Ц 6e = Cr2O72- +14H+
3 | S2O82- + 2e = 2SO42-
2. ’ромат(V≤)- або дихромат(V≤)-≥они у кислому роз≠чин≥ окиснюють
йодид-≥он, в≥дновлюючись, до хром(≤≤≤)- кат≥она:
6I- + —r2O72- + 14Ќ+ = 3≤2 + 2—r3+ + 7Ќ2O
3 | 2I- - 2е = ≤2
1 | —r2O72- + 6e + 14Ќ+ = 2—r3+ + 7Ќ2O
4. јріентум(≤)-, бар≥й- ≥ плюмбум(≤≤)-кат≥они осаджу≠ють хромат (V≤)-≥он з розчин≥в, утворюючи забарвлен≥ осади:
2јg+ + —rќ42- → јg2—rO4↓
¬а2+ + —rO42- → ¬а—rO4↓
–b2+ + —rO42- → –b—rќ4↓
–еакц≥њ виконують краплинним методом.
9. ≈ƒ“ј - Nа2Ќ2Y∙2Ќ2O - динатр≥й етилендиам≥нтетраацетат-вода(1/2) з хром(≤≤≤)-кат≥оном у слабкокислому се≠редовищ≥ утворюЇ координац≥йну сполуку ф≥олетового коль≠ору —rY- де Y4- - ан≥он ≈ƒ“ј:
—r3+ + Ќ2Y2- → —rY- + 2Ќ+

”мови реакц≥њ:
а) рЌ 4-5; б) надлишок ≈ƒ“ј; в) в≥дсутн≥сть Fе3+-, —u2+-, —о2+-, Ni2+-≥он≥в тощо, €к≥ мають хромофорн≥ власти≠вост≥; г) в≥дсутн≥сть оксалат- ≥ цитрат-ан≥он≥в; д) нагр≥ванн€.
1.√≥дрол≥з гексаг≥дроксоалюм≥нат≥в(≤≤≤) у присутност≥ амон≥й хлориду
NЌ4—1 за нагр≥ванн€:
[ј1(ќЌ2)6]3+ + 3ќЌ- → [ј1(ќЌ2)3OH)3]↓ + 3H2O
[ј1(ќЌ2)3OH)3] + 3ќЌ- → [ј1(ќЌ)6]3- + 3H2O
[ј1(ќЌ)6]3- + NH4+ + 2H2O → [ј1(ќЌ2)3OH)3]↓ + NH3↑ + 3H2O
2. јл≥зарин (H2Alis, 1,2-диокс≥антрах≥нон, —12Ќ6(—ќ)2(ќЌ)2) з амюм≥н≥й-кат≥оном утворюЇ червоний Ђалюм≥н≥Ївий лакї, €кий не розчин€Їтьс€ в ацетатн≥й, або в етанов≥й, кислот≥):
Al3+ + H2Alis + 3OH- → Al(OH)2(HAlis) + H2O

јл≥зарин
- 8-ќксих≥нол≥н (Ќќх≥n) з алюм≥н≥й-кат≥оном утворюЇ не≠розчинний у вод≥, але добре розчинний у орган≥чних розчинни≠ках алюм≥н≥й оксих≥нол€т солом'€но-жовтого кольору
÷инк(≤≤)-кат≥он
1. 4[Fе(CN)6]4- з Zn2+-≥оном утворюЇ б≥лий осад, €кий не розчин€Їтьс€ в розведених кислотах, але розчин€Їтьс€ в лугах:
3Zn2+ + 2 + + 2[Fе(—N)6]4- → 2Zn3[Fе(—N)6]2↓
2. 3[Fе(—N)6] з Zn2+-кат≥оном утворюЇ осад коричнево-жовтого кольору, €кий розчин€Їтьс€ в лугах:
3Zn2+ + 2[Fe(CN)6]3- → Zn3[Fe(CN)6]2↓
Ќ2S з Zn2+-кат≥оном у присутност≥ натр≥й ацетату утво≠рюЇ б≥лий драглистий осад, €кий не розчин€Їтьс€ в —Ќ3—ќќЌ:
Zn2+ + Ќ2S + 2—Ќ3—ќќ- → ZnS↓ + 2—Ќ3—ќќЌ
4. ƒитизон (дифен≥лт≥окарбазон, C6H5-NH-NH-CS-N=N-C6H5, H2Dz)
Ќ2Dz + 2OЌ- + 2Nа+ → Na2Dz + 2Ќ2O
—танум(≤≤)-кат≥он
2. Ѕ≥смут(≤≤≤)-кат≥он у лужному середовищ≥ окиснюЇ станум(≤≤)-кат≥он до станум(≤V)-катiона, в≥дновлюючись до оксами≠тово-чорного метал≥чного б≥смуту:
Sn2+ + 2OЌ- → Sn(ќЌ)2↓
Sn(ќЌ)2 + 2OЌ-  [Sn(ќH)4]2-
[Sn(ќH)4]2-
3[Sn(ќЌ)4]2- + 2¬≥(ќЌ)3 → 3[Sn(ќЌ)6]2- + 2¬i↓
6. Ќ2S - г≥дроген сульф≥д або Nа2S - натр≥й сульф≥д з Sn2+- ≥оном утворюЇ темно-коричневий осад SnS, €кий не розчин€Їтьс€ в надлишку реактиву (Ќ2S чи Nа2S).
Sn2+ + Ќ2S → SnS↓ + 2Ќ+
хлоридн≥й кислот≥ губчасте олово може знову розчинитис€.
5. ќксалатна (щавлева) кислота - Ќ2—2O4∙2Ќ2O з нейтраль≠ного або слабкокислого розчину станум(≤≤)-кат≥она осаджуЇ станум(≤≤) оксалат:
Sn2+ + Ќ2—2O4 → SnC2O4↓ + 2Ќ+
—танум(IV)-кат≥он
1. √≥дрол≥з солей станум(IV)-кат≥она
Ќайповн≥ше Sn—l4 г≥дрол≥зуЇ за нагр≥ванн€ ≥ у присутност≥ н≥тратних(V) або сульфатних(V≤) солей:
[Sn—l6]2- + 4ЌќЌ → Sn(ќЌ)4 ↓+ 4Ќ+ + 6—l-
3.Ќ2S - сульф≥дна кислота з Sn(≤V)-кат≥оном утворюЇ жовтий осад:
[Sn—l6]2- + 2Ќ2S → SnS2↓ + 4Ќ+ + 6—l-
ќсад розчин€Їтьс€ в концентрован≥й хлоридн≥й кислот≥, в лугах ≥ Na2S:
SnS2 + 6Ќ—lконц → Ќ2[Sn—l6] + 2Ќ2S↑
Ѕ≥лет 5
1. »—Ћќ“Ќќ-ќ—Ќќ¬Ќ≈ “»“–”¬јЌЌя (метод нейтрал≥зац≥њ)
титриметричний метод анал≥зу, в основ≥ €кого (у водному середовищ≥) лежить реакц≥€ нейтрал≥зац≥њ: H3O+ + OH-  2H2O.
2H2O.
–еакц≥њ кислотно-основноњ взаЇмод≥њ характеризуютьс€ великою швидк≥стю ≥ проход€ть строго стех≥ометрично.
—тандартними розчинами методу Ї 0,1 ћЕ0,001 ћ розчини HCl, H2SO4, NaOH, KOH. якщо €к титранти використовують розчини кислот, то метод називають ацидиметр≥Їю, а €кщо основи Ч алкал≥метр≥Їю.
1) јцидиметричне титруванн€:
- “итрують сильн≥ основи (наприклад, NaOH, KOH, Ba(OH)2 тощо):
OH- + H+ = H2O
- “итрують слабк≥ основи (наприклад, NH3H2O):
NH3H2O + H+ = NH4+ + H2O
- “итрують сол≥ неорган≥чних ≥ орган≥чних кислот, €к≥ г≥дрол≥зують з утворенн€м ќЌ- йон≥в (наприклад, Na2CO3, NaHCO3, CH3COONa тощо):
—ќ32- + HOH = HCO3- + OH-
H+ + OH- = H2O
¬ алкал≥метричному титруванн≥ титрантами Ї водн≥ розчини NaOH ≥ KOH з мол€рною концентрац≥Їю речовини екв≥валента 1 моль/дм3, 0,5 моль/дм3, 0,1 моль/дм3, 0,01 моль/дм3 (fекв (ћќЌ) = 1). –≥дше використовують розчини бар≥й диг≥дроксиду Ba(OH)2 (fекв (Ba(OH)2 = ½).
2) јлкал≥метричне титруванн€:
- “итрують сильн≥ кислоти (наприклад, HCl, H2SO4, HNO3 тощо):
H+ + OH- = H2O
- “итрують слабк≥ кислоти (наприклад, ортофосфатна(V), ацетатна, тартратна, оксалатна тощо):
H3PO4 + OH- = H2PO4- + H2O (≥ндикатор метилоранж)
- “итрують сол≥, €к≥ г≥дрол≥зують з утворенн€м г≥дроген (1+)-йон≥в (наприклад, NH4Cl тощо):
NH4+ + H2O = NH3H2O + H+, H+ + OH-= H2O
- “итрують г≥дроген-сол≥ (наприклад, NaH2PO4, Na2HPO4 тощо):
H2PO4- = HPO42- + H+
H2PO4- + OH- = HPO42- + H2O (≥ндикатор фенолфталењн)
2HPO42- + 2OH- + 3—а2+ = Ca3(PO4)2↓ + 2H2O (≥ндикатор фенолфталењн)
¬икористовують вторинн≥ стандартн≥ розчини; њх точну мол€рну концентрац≥ю встановлюють за стандартними речовинами або при њх титруванн≥ розчинами в≥домоњ концентрац≥њ. —тандартизац≥ю розчин≥в кислот провод€ть за стандартними речовинами: натр≥ю тетраборатом (Na2B4O7⋅10H2O), натр≥ю карбонатом (Na2CO3) або за стандартними розчинами луг≥в (NaOH та KOH). —тандартизац≥ю розчин≥в луг≥в провод€ть за стандартними речовинами: оксалатною кислотою (H2C2O4), бурштиновою кислотою (H2C4H4O4), €нтарною, за стандартними розчинами HCl, H2SO4.
ѕрийоми кислотно-основного титруванн€: пр€ме, зворотне, титруванн€ ≥з зам≥сником.
—пособи виконанн€ операц≥й: п≥петкуванн€, окремих наважок.
” методах кислотно-основного титруванн€ “≈ (“≈ Ц момент, коли речовини прорегували м≥ж собою в точних екв≥валентних к≥лькост€х) не супроводжуЇтьс€ видимим зовн≥шн≥м ефектом ≥ може лежати в нейтральному, лужному ≥ кислому середовищ≥. “ому дл€ визначенн€ “≈ використовують кислотно-основн≥ ≥ндикатори.
≤ндикатори Ц це орган≥чн≥ сполуки, €к≥ зм≥нюють свою будову ≥ ф≥зичн≥ властивост≥ (кол≥р) залежно в≥д зм≥ни величини рЌ середовища. “ому так≥ ≥ндикатори називають рЌ-≥ндикаторами (наприклад, лакмус, фенолфталењн, метиловий оранжевий, метилчервоний).
¬ипадки титруванн€:
1) “итруванн€ сильноњ кислоти сильною основою (чи навпаки);
2) “итруванн€ слабкоњ кислоти сильною основою (чи навпаки);
3) “итруванн€ слабкоњ основи сильною кислотою.
ѕриклади визначень:
- јцтдиметр≥€
1. ¬изначенн€ вм≥сту натр≥й г≥дроксиду у розчин≥ нев≥домоњ концентрац≥њ способом п≥петкуванн€.
2. ¬изначенн€ вм≥сту натр≥й г≥дроксиду ≥ натр≥й карбонату при сп≥льн≥й њх присутност≥.
3. ¬изначенн€ вм≥сту амон≥аку у водному розчин≥ його
4. ¬изначенн€ вм≥сту натр≥й карбонату ≥ натр≥й г≥дроген карбонату при сп≥льн≥й њх присутност≥ способом п≥петкуванн€.
5. ¬изначенн€ карбонатноњ(тимчасовоњ) твердост≥ води.
- јлкал≥метр≥€
1. ¬изначенн€ вм≥сту г≥дроген хлориду ≥ триг≥дроген триоксоборату при сп≥льн≥й њх присутност≥ у розчин≥.
2. ¬изначенн€ кислотност≥ хл≥ба.
3. ¬изначенн€ кислотност≥ молока.
4. ¬изначенн€ вм≥сту ацетатноњ (етановоњ) кислоти у зразках њњ.
5. ¬изначенн€ загального вм≥сту фосфору в суперфосфат≥.
6. ¬изначенн€ вм≥сту кал≥ю в кал≥йних добривах.
2. ат≥они четвертоњ анал≥тичноњ групи Ц Cr3+, Al3+, Zn2+,Sn2+,Sn4+,As3+,
As5+- утворюють х≥м≥чн≥ елементи р≥зних груп ≥ п≥дгруп пер≥одичноњ таблиц≥; утворюють типов≥ амфотерн≥ елементи.
Ѕлизьк≥ електронна будова ≥ рад≥ус кат≥он≥в четвертоњ анал≥тичноњ групи обумовлюють однакове в≥дношенн€ њх до сильних основних г≥дроксид≥в Ц луг≥в.
ƒ≥€ групового реактиву NaOH або KOH (NH4OH):
ћеn+ + nOH- = Me(OH)n↓
Zn2+ + 2ќЌ- = Zn(ќЌ)2↓ (б≥лий); Al3+ + 3OH- = Al(OH)3↓ (б≥лий);
Zn(ќЌ)2↓ + 2ќЌ- = [Zn(OH)4]2-; Al(OH)3↓ + 3OH- = [Al(OH)6]3-.
1) —ол≥ Cr3+ мають зелене або синьо-ф≥олетове забарвленн€.
ќкисненн€ Cr3+ до Cr6+ р≥зними окисниками:
2Cr3+ + 3H2O2 + 10OH- = 2CrO42- +8H2O (жовтий кол≥р)
2CrCl3 +3H2O2 + 10NaOH = 2Na2CrO4 +8H2O + 6NaCl.
10Cr3+ + 6MnO4- +11H2O = 5Cr2O72- + 6Mn2+ + 22H+(жовто-оранжевий)
2Cr2(SO4)3 + 6KMnO4 +11H2O = 5H2Cr2O7 + 6MnSO4 +3K2SO4 +6H2SO4.
2Cr3+ + 3S2O82- + 7H2O =AgNO3 Cr2O72- + 6SO42- + 14H+
Cr2(SO4)3 + 3(NH4)2S2O8 + 7H2O =AgNO3 (NH4)2Cr2O7 + 3(NH4)2SO4 + 7H2SO4.
2) Al3+ + H2Alis + 3OH- =Al(OH)2(HAlis) + H2O (червоний Ђалюм≥н≥Ївийї лак)
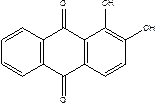 ал≥зарин
ал≥зарин 
3) —ол≥ Zn2+ безбарвн≥, б≥льш≥сть з них розчинн≥ у вод≥.
3Zn2+ + 2 + + 2[Fe(CN)6]4- = K2Zn3[Fe(CN)6]2↓(б≥лий)
3ZnSO4 + 2 4[Fe(CN)6] = K2Zn3[Fe(CN)6]2↓ + 3K2SO4
Zn2+ + S2- = ZnS↓(б≥лий)
ZnCl2 + (NH4)2 S = ZnS↓+ 2NH4Cl
4) (Sn2+,Sn4+,As3+,As5+) + H2S =
SnS↓ - осад темно-коричневого кольору;
SnS2↓- жовтий;
As2S3↓(As2S5↓) - осад св≥тло-жовтого кольору;
5) ћагнез≥альна сум≥ш (MgCl2 + NH3 H2O + NH4Cl) на As5+:
Mg2+ + NH4+ + AsO43- = MgNH4AsO4↓(б≥лий кристал≥чний осад).
ћол≥бденова р≥дина в кислому середовищ≥ на As5+:
H3AsO4 + 12MoO42- + 3NH4+ +21H+ = (NH4)3[As(Mo3O10)4] 2H2O↓ +10H2O (жовтий кристал≥чний осад).
3. √етерогенна система Ц це система,€ка складаЇтьс€ з к≥лькох фаз.
Ќайпрост≥шою гетерогенною системою Ї малорозчинний електрол≥т, що утворюЇ насичений розчин, €кий складаЇтьс€ з осаду та розчину, €к≥ перебувають у стан≥ х≥м≥чноњ р≥вноваги:
¬аSO4↓ = BaSO4 = Ba2+ + SO42- - насичений розчин малорозчинного електрол≥та.
‘актори, що впливають на розчинн≥сть речовин:
1) Ќа€вн≥сть однойменних йон≥в ≥ сильних електрол≥т≥в;
2) “емпература;
3) рЌ- середовище;
4) ѕрирода розчинника;
5) ѕрисутн≥сть комплексоутворювач≥в, окисник≥в, або в≥дновник≥в.
ƒобуток розчинност≥ Ц це добуток мол€рних концентрац≥й йон≥в у насиченому розчин≥ малорозчинного електрол≥ту за стлоњ температури ≥ тиску.
“аким чином, малорозчинн≥ речовини Ц це сильн≥ електрол≥ти, оск≥льки у водних розчинах ≥снують у вигл€д≥ йон≥в.
‘≥зичний зм≥ст р≥вн€нн€ ƒ–: незалежно в≥д зм≥ни мол€рноњ концентрац≥њ окремих йон≥в у розчин≥ значенн€ ƒ– завжди залишаЇтьс€ сталим за сталоњ температури.
ƒ– (BaSO4) = [Ba2+][SO42-]
ƒобуток активност≥: ƒј = ƒ–/y2, у Ц коеф≥ц≥Їнт активност≥ в≥дпов≥дного малорозчинного електрол≥та.
ƒј не залежить в≥д концентрац≥њ, сторонн≥х йон≥в, в≥д температури.
ѕравило ƒ–: осад малорозчинного електрол≥та осаджуЇтьс€ тод≥, коли дос€гнуто ≥ перевищено величину його добутку розчинност≥.
- « п≥двищенн€м температури розчинн≥сть осад≥в зб≥льшуЇтьс€;
- ѕри зб≥льшенн≥ –Ќ (зниженн≥ рќЌ) розчинн≥сть осад≥в зб≥льшуЇтьс€.
ƒробне осадженн€: електрол≥т, €кий маЇ найнижчий ƒ– осаджуЇтьс€ першим. ѕри близьких значенн€х ƒ– можливе утворенн€ дек≥лькох осад≥в малорозчинних електрол≥т≥в ≥ зд≥йснюЇтьс€ пропорц≥йно до величини ƒ–.
4.
| ƒано: m(наважки) = 0,2017г. m(тигл€ з Fe2O3) = 10,4954г. m(порожнього тигл€) = 10,2124г. | –озвТ€занн€: 1) яка маса грав≥метричноњ форми? m (Fe2O3) =10,4954 Ц 10,2124 = 0,283г. 2) яка масова частка (у%) ‘еруму у проб≥? W (%)(Fe) = [m(Fe2O3) х [F(2M(Fe)/M(Fe2O3))] х 100%/ m(наважки) = 0,283г. х 0,6994 х 100%/ 0,2017г. = 98,130 |
W (%)(Fe) -?
¬≥дпов≥дь: W (%)(Fe) = 98,13
Ѕ≥лет є 6
1. “итруванн€ сильноњ кислоти сильною основою, слабкоњ кислоти сильною основою, слабкоњ основи сильною кислотою. –озра






