—лабк≥ кислоти Ц розчинен≥ у вод≥, частково дисоц≥юють на ≥они, тому можна допустити, що концентрац≥€ недисоц≥йованих молекул кислоти практично дор≥внюЇ загальн≥й концентрац≥њ кислоти:
[HA] = Ck
pH = ½ pKk - lgCk, де pKk Цлогарифм константи дисоц≥ац≥њ кислоти з в≥дТЇмним знаком. ƒл€ 1 н. розчину слабкоњ кислоти Ck=1, а lg 1=0, формула дл€ обчисленн€ рЌ розчину 1 н. слабкоњ кислоти маЇ вигл€д:
рЌ= ½ pKk
« р≥вн€нн€ вит≥каЇ, що ≥з зм≥ною концентрац≥њ кислоти зм≥нюЇтьс€ рЌ розчину.
—лабк≥ основи. онцентрац≥ю г≥дроксильних йон≥в слабих основ рќЌ обчислюють так само, €к ≥ рЌ слабких кислот:
рќЌ = 1/2р о = ½ lg—о
знаючи рќЌ, знаход€ть рЌ за р≥вн€нн€м рЌ = 14-рќЌ.
ƒано:
V(р-ну)=2дм
“(KMnO4/Na2C2O4)=0,008348г/см
m (наважки)=?
______________________________________
m (KMnO4)=c 1/5 KMnO4 *V (Na2C2O4)/1000
T=c* Me/1000 c (1/5 KMnO4 )=T*1000/ Me Me=1/5*158.036=31.6
c (1/5 KMnO4 )= 0,008348г/см*1000/31.6=0.2641
m (KMnO4)=0.2641*2/1000=0.00053
3.¬изначенн€ вм≥сту йон≥в купруму (II) методом йодометричного титруванн€
упрум (II) взаЇмод≥Ї з кал≥й йодидом, окиснюючи до в≥льного йоду,к≥льк≥сть речовини €кого встановлюють титруванн€м стандартним стандартним розчином Na2S2O3 * 5 H2O
4 KI + 2 CuSO4 = I2 +2 CuI + 2 K2 SO4
2 Na2S2O3 + I2 = Na2S4O6 + 2 NaI
ѕри йодометричному титруванн≥ ввизначенн≥ купруму використовують метод титруванн€ зам≥сника
m (наважки)=ћ(CuSO4 * 5 H2O)* с (CuSO4 * 5 H2O)* V ќЋЅ»/1000
Ѕ≥лет 20
1. «агальним принципом майже вс≥х метод≥в розд≥ленн€ ≥ концентруванн€ Ї
використанн€ процес≥в розпод≥лу речовини м≥ж двома фазами, причому анал≥зована речовина зосереджуЇтьс€ в одн≥й з фаз: р≥дк≥й, тверд≥й чи газопод≥бн≥й. ƒл€ розд≥ленн€ ≥ концентруванн€ елемент≥в ≥снуЇ багато р≥зних метод≥в, серед €ких одне з перших м≥сць займають хроматограф≥чн≥ методи. ¬елике значенн€ також маЇ осадженн€ з водних ≥ неводних розчин≥в орган≥чними ≥ неорган≥чними осаджувачами, електрол≥тичне осадженн€, сп≥восадженн€, екстрагуванн€ ≥ в≥дг≥н летких речовин. ’роматограф≥€ Ц це спос≥б розд≥ленн€ речовин, заснований на розходженн≥ в њхн≥х коеф≥ц≥Їнтах розпод≥лу м≥ж двома фазами, одна з €ких нерухома, а ≥нша направлено рухаЇтьс€ щодо першоњ (уздовж стовпчика чи тонкого шару нерухомоњ фази). ≈кстракц≥€ Ц найб≥льш важливий ≥ розповсюджений метод концентруванн€. ¬≥н в≥др≥зн€Їтьс€ ун≥версальн≥стю, придатний ≥ дл€ скиданн€ матриц≥, ≥ дл€ в≥дд≥ленн€ м≥крокомпонент≥в, забезпечуЇ досить високу ефективн≥сть концентруванн€. Ќедол≥к методу - в≥дносно невисокий ступ≥нь концентруванн€; правда, екстракц≥йна хроматограф≥€ забезпечуЇ дуже високий ступ≥нь абсолютного концентруванн€. ≥льк≥сно екстракц≥ю можна описати за допомогою константи розпод≥лу:
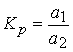 , де а1 ≥ а2 Ч активност≥ речовини, розпод≥леноњ в орган≥чн≥й ≥ водн≥й фазах.
, де а1 ≥ а2 Ч активност≥ речовини, розпод≥леноњ в орган≥чн≥й ≥ водн≥й фазах.
ћетод осадженн€ застосовують дл€ б≥льш грубого под≥лу елемент≥в. ѕри його застосуванн≥ завжди спостер≥гаютьс€ втрати внасл≥док розчинност≥ осад≥в, часто заважаЇ сп≥восадженн€. ќсадженн€ дозвол€Ї т≥льки перерозпод≥лити елементи м≥ж розчином ≥ осадом, кожний з €ких завжди м≥стить сум≥ш ус≥х присутн≥х елемент≥в.
|
|
|
—п≥восадженн€, засноване на вид≥ленн≥ в осад м≥крокомпонент≥в з орган≥чним або неорган≥чним колектором, не знайшло наст≥льки широкого поширенн€, €к екстракц≥€, через б≥льшу трудом≥стк≥сть ≥ тривал≥сть. ћ≥крокомпоненти часто вид≥л€ютьс€ не повн≥стю. ќднак по ступен≥ абсолютного концентруванн€, простот≥ й апаратурному оформленню сп≥восадженн€ Ї одним ≥з кращих метод≥в. Ќе сл≥д в≥ддавати перевагу тому чи ≥ншому методу анал≥зу, не беручи до уваги характер досл≥джуваного об'Їкта, його призначенн€, агрегатний стан, концентрац≥ю, на€вн≥сть дом≥шок, мети анал≥зу, необх≥дну точн≥сть, терм≥н виконанн€ анал≥зу ≥ т. ≥н.
ƒл€ опису концентруванн€ використовують так≥ к≥льк≥сн≥ характеристики:
- ступ≥нь вилученн€
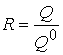 ;
;
- коеф≥ц≥Їнт концентруванн€
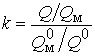 ;
;
- коеф≥ц≥Їнт розд≥ленн€
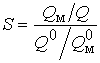 , де Q0 ≥ Q Ц к≥льк≥сть м≥кроелемента в зразку ≥ в концентрат≥; Q0м ≥ Qм Ц к≥льк≥сть матриц≥ до ≥ п≥сл€ концентруванн€ в≥дпов≥дно.
, де Q0 ≥ Q Ц к≥льк≥сть м≥кроелемента в зразку ≥ в концентрат≥; Q0м ≥ Qм Ц к≥льк≥сть матриц≥ до ≥ п≥сл€ концентруванн€ в≥дпов≥дно.
2. «акон д≥ючих мас встановлюЇ сп≥вв≥дношенн€ м≥ж масами реагуючих речовин в
х≥м≥чних реакц≥€х при р≥вноваз≥, а також залежн≥сть швидкост≥ х≥м≥чноњ реакц≥њ в≥д концентрац≥њ вих≥дних речовин. Ўвидк≥сть х≥м≥чноњ реакц≥њ пр€мо пропорц≥йна добутку концентрац≥й реагуючих речовин. “ак, дл€ реакц≥њ ј + ¬ = — маЇмо u = k [A] [B], де u - швидк≥сть; k - коеф≥ц≥Їнт пропорц≥йност≥ (константа швидкост≥); [A] ≥ [B] - концентрац≥њ речовин ј ≥ ¬.
1. якщо у р≥вноважну систему при стал≥й температур≥ ввести додаткову к≥льк≥сть речовини, то стан р≥вноваги порушуЇтьс€, ≥ реакц≥€ знову починаЇтьс€; стан р≥вноваги встановитьс€ при т≥й сам≥й констант≥ р≥вноваги.
2. ѕри додаванн≥ у р≥вноважну систему початкових продукт≥в реакц≥њ або при зменшенн≥ к≥нцевих њњ продукт≥в р≥вновага зм≥щу-Їтьс€ вправо; при додаванн≥ к≥нцевих продукт≥в реакц≥њ або зме-ншенн≥ початкових њњ продукт≥в р≥вновага зм≥щуЇтьс€ вл≥во.
3. ѕ≥д час проходженн€ процесу швидк≥сть пр€моњ реакц≥њ зме-ншуЇтьс€, а швидк≥сть зворотноњ реакц≥њ зб≥льшуЇтьс€.
4. якщо константа р≥вноваги зростаЇ, то р≥вновага зм≥щуЇтьс€ вправо, вих≥д к≥нцевих продукт≥в реакц≥њ зростаЇ, ≥ навпаки.
«а теор≥Їю сильних електрол≥т≥в сильн≥ електрол≥ти у розбавлених водних розчинах практично дисоц≥юють повн≥стю. ѕричиною такоњ дисоц≥ац≥њ сильних електрол≥т≥в Ї йон-дипольна взаЇмод≥€ сильного електрол≥ту з молекулами води.
ƒо сильних електрол≥т≥в в≥дноситьс€ б≥льша частина солей, €к≥ мають йонну кристал≥чну структуру, ≥ розведен≥ водн≥ розчини кислотних ≥ основних г≥дроксид≥в.
” водних розчинах електрол≥т≥в ви€вл€ютьс€ лише г≥дратован≥ кат≥они ≥ ан≥они. Ќаприклад, у водному розчин≥ купрум дихлориду Ц CuCl2 присутн≥ [Cu(OH)6]2-, [Cl(H2O)n], H3O+ тощо.
” водних розчинах сильних електрол≥т≥в м≥ж йонами Ї електростатична взаЇмод≥€ та ≥нш≥ взаЇмод≥њ, що обумовлюЇ меншу рухлив≥сть йон≥в, а отже, ≥ активн≥сть њх. јктивн≥сть враховуЇ взаЇмне прит€ганн€ йон≥в, взаЇмод≥ю розчиненоњ речовини з структурними елементами розчинника, присутн≥сть ≥нших електрол≥т≥в, €к≥ зм≥нюють рухлив≥сть йон≥в у розчин≥.
јктивн≥сть дл€ безк≥нечно розбавлених водних розчин≥в дор≥внюЇ мол€рн≥й концентрац≥њ речовини у розчин≥: а(’) = с(’), моль/дм3.
” реальних розчинах активн≥сть йон≥в менша, н≥ж мол€рна концентрац≥€ речовини у розчин≥, причиною цього Ї сильна м≥ж≥онна взаЇмод≥€. ƒл€ врахуванн€ цього вводитьс€ пон€тт€ про коеф≥ц≥Їнт активност≥ йона:
|
|
|
fx= a(X) / [X]
…онна сила розчину Ї м≥рою електростатичноњ взаЇмод≥њ м≥ж йонами ≥ визначаЇтьс€ р≥вн€нн€м:
M = 1/2 ∑c(Xi)∙ z2Xi, моль/дм3
1. ƒл€ розбавлених розчин≥в електрол≥т≥в типу +ј-(KCl, NaNO3)^
M = c(KA) = c(X)
2. ƒл€ розбавлених розчин≥в електрол≥т≥в типу +ј2-(K2S, Na2S) i K2+A
(ZnCl2, CaCl2): M = 3c(X)
3. ƒл€ розбавлених розчин≥в електрол≥т≥в типу K2+A2- (ZnSO4, MnSO4):
ћ = 4с(’)
” розчинах слабких електрол≥т≥в йонна сила дор≥внюЇ нулю.
јлгоритм обчисленн€ активност≥ йона у розчин≥ Ц а(’):
1) –озрахунок йонноњ сили розчину - ћ(’);
2) –озрахунок коеф≥ц≥Їнт≥в активност≥ - fx
3) ќбчисленн€ активност≥ йона: а(’) =[X]∙ fx
3.‘отометричне визначенн€ вм≥сту феруму за методом кал≥брувального граф≥ка.
—уть методу калбрувального граф≥ка пол€гаЇ в тому, що при л≥н≥йн≥й залежност≥ м≥ж двома параметрами, при досл≥дженн≥ стандартних зразк≥в можна одержати реперн≥ точки, побудувати граф≥к, €кий зТЇднуЇ ц≥ точки. ѕот≥м, використовуючи цей граф≥к, можна знайти значенн€ в пром≥жних точках.
ƒл€ побудови кал≥брувального граф≥ка беретьс€ ш≥сть розчин≥в ≥ зам≥рюютьс€ њх оптичн≥ величини. ƒал≥ будуЇтьс€ кал≥брувальний граф≥к
ј= 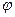 (—) ≥ ј=
(—) ≥ ј= 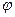 (“), оптична густина Ї залежн≥стю в≥д концентрац≥њ або в≥д титру. √раф≥к повинен бути пр€мол≥н≥йним. ≤ тод≥ зам≥р€вши јх на основ≥ граф≥ка спустивши на в≥сь х (абсцис) перпендикул€рно одержують цю концентрац≥ю.
(“), оптична густина Ї залежн≥стю в≥д концентрац≥њ або в≥д титру. √раф≥к повинен бути пр€мол≥н≥йним. ≤ тод≥ зам≥р€вши јх на основ≥ граф≥ка спустивши на в≥сь х (абсцис) перпендикул€рно одержують цю концентрац≥ю.
4.
| ƒано: m(наважки) = 0,2254г. Vколби = 100см3 V(досл≥дж. розчину) = 10см3 V(AgNO3) = 18см3 c(AgNO3) = 0,0204 моль/дм3 V(NH4SCN) = 5,78 см3 c(NH4SCN) = 0,0458 моль/дм3 | —l- + Ag+надлишок = AgCl↓+ Ag+ Ag+зал + SCN-титрант = AgSCN↓ W (%)(Cl-) = ME(Cl-) ∙(c(AgNO3) ∙ V(AgNO3) Ц c(NH4SCN) ∙ V(NH4SCN))∙ Vколби ∙ 100 / 1000 ∙ m(наважки) ∙ 10 = 35,45 г/моль ∙ (0,0204 моль/дм3 ∙ 18 см3 Ц 0,0458 моль/дм3 ∙ 5,78 см3) ∙ 100см3 ∙ 100 / 1000∙ 0,2254 г. ∙10см3 = 16,117% |
W(%)(Cl-) -?
¬≥дпов≥дь: W(%)(Cl-) = 16,117%
Ѕ≥лет 21
1. —орбц≥€-процес поглинанн€ газ≥в, пар≥в та розчинених речовин твердими або р≥дкими поглиначами на твердому нос≥њ (сорбентами). ласиф≥кац≥€ сорбц≥йних метод≥в заснована на р≥зниц≥ механ≥зму взаЇмод≥њ речовин з сорбентами. –озр≥зн€ють абсорбц≥ю (хемосорбц≥€) ≥ адсорбц≥ю (ф≥зична адсорбц≥€), розпод≥л речовин м≥ж двома несмешивающимис€ фазами (розчинник ≥ р≥дка фаза на сорбент≥) ≥ кап≥л€рну конденсац≥ю - утворенн€ р≥дкоњ фази в порах ≥ кап≥л€рах твердого сорбенту при поглинанн≥ пари речовини. ” чистому вигл€д≥ кожний з перерахованих механ≥зм≥в, €к правило, не реал≥зуЇтьс€, ≥ зазвичай спостер≥гаютьс€ зм≥шан≥ механ≥зми. “аким чином, сорбц≥йн≥ процеси р≥зн≥ за њх механ≥зму. ѕроте будь-€кий сорбц≥йний процес починаЇтьс€ з адсорбц≥њ на кордон≥ дотичних фаз, €к≥ можуть бути р≥дкими, газопод≥бними або твердими.
—орбц≥€ може проходити в статичних або динам≥чних умовах. —орбц≥ю називають статичною, коли поглинаЇтьс€ речовина (сорбт≥в), що знаходитьс€ в газопод≥бному фаз≥ або р≥дкоњ фаз≥, наведено в контакт з нерухомим сорбентом або перем≥шуЇтьс€ з ним. —татичну активн≥сть сорбенту характеризують к≥льк≥стю поглиненоњ речовини на одиницю маси сорбенту в певних умовах.
ƒинам≥чноњ сорбц≥ю називають у тому випадку, коли поглинаЇтьс€ речовина знаходитьс€ в рухом≥й або газопод≥бн≥й фаз≥, €ка ф≥льтруЇтьс€ через шар сорбенту. ƒинам≥чну активн≥сть адсорбенту характеризують часом в≥д початку пропусканн€ адсорбт≥ва до його проскакуванн€, тобто до по€ви його за шаром адсорбенту
јктивне вуг≥лл€ - непол€рн≥ сорбенти з сильно розвиненою пористою структурою. ѓх отримують з карбоновм≥сних речовин шл€хом високотемпературного окисленн€ в струмен≥ вод€ноњ пари або х≥м≥чною обробкою, наприклад, фосфорноњ або с≥рчаноњ кис лотами. јктивне вуг≥лл€ мають пори р≥зного розм≥ру: м≥кропори з д≥аметром 1-2 њм, пори перех≥дного розм≥ру (5 - 50 њм) ≥ макропори (понад 100 њм). ѕитома поверхн€ м≥кропор складаЇ 1000 1800 м '/ р. макропор - близько 1 м2 / г. ” газов≥й хроматограф≥њ застосовуютьс€ вуг≥лл€ з максимально розвиненою поверхн≥стю м≥кропор.
јктивне вуг≥лл€ виб≥рково адсорбують вуглеводн≥ та њх пох≥дн≥, ароматичн≥ сполуки, барвники, слабк≥ше - нижч≥ спирти, карбонов≥ кислоти, складн≥ еф≥ри.
|
|
|
ремнезем (S≥ќn-хЌ2ќ) - це сухий гель сил≥ц≥Ївоњ кислоти з аморфною структурою, г≥дроф≥льний сорбент з розвиненою пористою структурою. ” гранично г≥дроксильованому кремнезем≥ на поверхн≥ знаход€тьс€ 4,6-4,8 ќЌ- груп/нм2. Ќа€вн≥сть цих груп, що мають слабкокислотн≥ властивост≥, та њх нер≥вном≥рне розм≥щенн€ на поверхн≥ зумовлюють неоднор≥дн≥сть властивостей поверхн≥ адсорбенту.
ислий г≥дратований кремнезем (рЌ 3-5) називають також сил≥ц≥Ївою кислотою. Ќейтральний кремнезем використовують дл€ розд≥ленн€ нейтральних сполук або сполук, €к≥ ви€вл€ють основн≥ властивост≥, а дл€ розд≥ленн€ сполук з кислотними властивост€ми застосовують сил≥ц≥Їву кислоту. Ќерухом≥ фази на основ≥ кремнезему, особливо з модиф≥кованою поверхнею, Ї найпоширен≥шими сорбентами у високоефективн≥й р≥динн≥й ≥ газов≥й хроматограф≥њ. ‘≥рми, €к≥ випускають колонки дл€ хроматограф≥њ, розробл€ють сил≥кагель р≥зноман≥тних марок, д≥аметр зерен €ких вар≥юЇ в≥д 2 до 10 мкм, д≥аметр пор - в≥д 3 до 75 нм, в≥дпов≥дно величина питомоњ поверхн≥ - в≥д «ќ до 700 м2/г. Ќедол≥ками кремнезему €к сорбенту дл€ хроматограф≥њ Ї: розчинн≥сть при рЌ менше 2 ≥ б≥льше 9; неоднор≥дн≥сть поверхневих силанольних груп, що призводить до викривленн€ форм п≥к≥в;
2. ƒл€ буферноњ системи 1-го типу, наприклад, ацетатноњ, концентрац≥ю ≥он≥в Ќ + у розчин≥ легко обчислить, виход€чи з константи кислотно-лужноњ р≥вноваги оцтовоњ кислоти:
≥льк≥сть речовини сильноњ кислоти або основи, €ка при додаванн≥ до буферного розчину викликаЇ зм≥ну рЌ на невелику певну величину, характеризуЇ буферну Їмн≥сть (–).
≥льк≥сно буферну Їмн≥сть визначають €к к≥льк≥сть речовини сильноњ основи або кислоти, €ку необх≥дно прилити до 1 дм3 розчину, щоб зм≥нити значенн€ його рЌ на одиницю. Ѕуферна Їмн≥сть розчину п≥двищуЇтьс€ ≥з зб≥льшенн€м к≥лькост≥ речовини буфера в систем≥. Ќайб≥льша буферна Їмн≥сть за даноњ концентрац≥њ буфера дос€гаЇтьс€ тод≥, коли спр€жен≥ кислота ≥ основа присутн≥ у розчин≥ буфера в екв≥мол€рних концетрац≥€х. Ѕуферна Їмн≥сть розчину залежить в≥д: 1) концентрац≥њ його компонент≥в; 2) природи речовини, тобто д кислоти чи основи; 3) сп≥вв≥дношенн€ концентрац≥й кислоти ≥ сол≥ чи основи ≥ сол≥.
ѕри введенн≥ до буферноњ сум≥ш≥ розчину сильноњ кислоти або сильноњ основи зм≥нюЇтьс€ концентрац≥€ слабкоњ кислоти або основи, проте рЌ буферного розчину практично не зм≥нюЇтьс€. ÷е по€снюЇтьс€ тим, що слабка кислота або слабка основа взагал≥ мало дисоц≥юЇ, а при на€вност≥ однойменних йон≥в њњ сол≥ дисоц≥ац≥€ буде ще менш в≥дчутною. –озведенн€ розчину не впливаЇ на його рЌ, оск≥льки водневий показник залежить лише в≥д сп≥вв≥дношенн€ концентрац≥й сол≥ (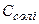 ) та кислоти (
) та кислоти (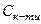 ) ≥ не залежить в≥д ступен€ розведенн€.
) ≥ не залежить в≥д ступен€ розведенн€.
ƒл€ кислого буферного розчину, утвореного слабкою кислотою та њњ с≥ллю, кислотн≥сть обчислюють за р≥вн€нн€ми
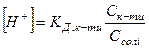 або
або 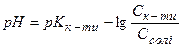 . (2.36)
. (2.36)
ƒл€ лужного буферного розчину, утвореного слабкою основою та њњ с≥ллю, кислотн≥сть розраховують за р≥вн€нн€ми
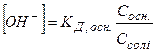 або
або 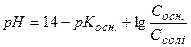 .
.
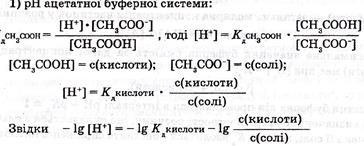

3. »онний добуток води. Ўкала рЌ ≥ рќЌ
” 1879 р н≥мецький ф≥зик ≥ ф≥зико-х≥м≥к ‘. ольрауш розробив метод, €кий дозвол€Ї встановлювати ступ≥нь дисоц≥ац≥њ електрол≥ту. ѕ≥зн≥ше, в 1894 р. за допомогою цього методу в≥н експериментально встановив, що вода (диг≥дроіен оксид)1- слабкий електрол≥т, дисоц≥ац≥€ €кого на г≥дроіен(1+)- ≥ г≥дроксид-≥они проходить за р≥вн€нн€м:
|
|
|
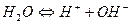 .
.
ƒл€ стану р≥вноваги константа дисоц≥ац≥њ води маЇ вигл€д
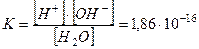 моль/л.
моль/л.
що за к≥мнатноњ температури в 1 дм3 води м≥ститьс€ 10-7 моль г≥дроіен(1+)- ≥ ё"7 моль г≥дроксид-≥он≥в.
ќтже, дисоц≥юЇ на йони всього 10~7 моль молекул Ќ20 у 1 дм3 води.
¬ода (Ќ20) ^ слабкий електрол≥т, тому с(Ќ20) = [Ќ20] = 55.5 моль/дм3. “аким чином, к≥льк≥сть речовини диг≥дроіен окси≠ду (Ќ20) 55.5 моль/дм3 можна вважати сталою величиною. –≥вн€нн€ (2.1) можна записати
 , (2.2)
, (2.2)
де 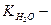 йонний добуток води.
йонний добуток води.
як би не зм≥нювались концентрац≥њ йон≥в 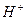 або
або 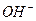 , њх добуток у будь-€кому водному розчин≥ Ї величиною сталою при к≥мнатн≥й температур≥. …онний добуток води залежить лише в≥д температури. « п≥двищенн€м температури зб≥льшуЇтьс€ йонний добуток води. ѕри
, њх добуток у будь-€кому водному розчин≥ Ї величиною сталою при к≥мнатн≥й температур≥. …онний добуток води залежить лише в≥д температури. « п≥двищенн€м температури зб≥льшуЇтьс€ йонний добуток води. ѕри 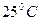
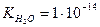 , а концентрац≥њ йон≥в г≥дрогену та г≥дроксиду однаков≥:
, а концентрац≥њ йон≥в г≥дрогену та г≥дроксиду однаков≥: 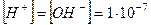 моль/л. —п≥вв≥дношенн€ м≥ж концентрац≥€ми йон≥в г≥дрогену та г≥дроксиду справедливе не лише дл€ чистоњ води, але й дл€ будь-€ких водних розчин≥в.
моль/л. —п≥вв≥дношенн€ м≥ж концентрац≥€ми йон≥в г≥дрогену та г≥дроксиду справедливе не лише дл€ чистоњ води, але й дл€ будь-€ких водних розчин≥в.
ќтже, йонний добуток води теж повинен зростати, що й п≥дтверджують експерименти.
« йонного добутку води виходить, що мол€рн≥ концентрац≥њ речовин г≥дроіен(1+)- ≥ г≥дроксид-≥он≥в зв'€зан≥ м≥ж собою ≥ обер≠нено пропорц≥йн≥ одна одн≥й. якщо вони р≥вн≥, то середовище нейтральне. ѕри додаванн≥ до води кислоти мол€рна концент≠рац≥€ речовини г≥дроіен(1+)-≥он≥в п≥двищуЇтьс€, а г≥дроксид- ≥он≥в понижуЇтьс€.
—. —еренсен запропонував практично виражати мол€рну концентрац≥ю речовини г≥дроіен(1+)-≥он≥в за допомогою дес€т≠кового логарифма, вз€того з оберненим знаком. ¬≥н назвав це число водневим показником ≥ позначив його символом рЌ. “ак, €кщо мол€рна концентрац≥€ речовини г≥дроіен(1+)-≥он≥в у нейт≠ральному розчин≥ дор≥внюЇ 1-ё"7 моль/дм3, то рЌ = -lgЌ+] = 7. рќЌ = -lg[ќЌ ] = 7. “од≥ рЌ + рќЌ = 14 ≥ рЌ = 14 - рќЌ чи навпаки.
ћетоди визначенн€ рЌ. ѕриблизне визначенн€ рЌ у водних розчинах провод€ть за допомогою х≥м≥чних ≥ндикатор≥в, €к≥ зм≥нюють св≥й кол≥р у певному ≥нтервал≥ значень рЌ. ѕри вико≠нанн≥ €к≥сного анал≥зу визначенн€ рЌ провод€ть з метою ви€с≠ненн€ кислотност≥ середовища, необх≥дного дл€ ви€вленн€ або розд≥ленн€ йон≥в. ќц≥нюють приблизне значенн€ рЌ за допомо≠гою лакмусового та ≥нших вид≥в ≥ндикаторного пап≥рц€.
Ћакмусовий пап≥рець - це смужка ф≥льтрувального паперу, €ка просочена розчином лакмусу, п≥дфарбованого невеликою к≥льк≥стю речовини кислоти у рожевий кол≥р або розчином лугу - в син≥й кол≥р. ѕри висушуванн≥ такого паперу ≥ одержуЇтьс€ "червоний або син≥й лакмусовий пап≥рець".
якщо при нанесенн≥ на син≥й лакмусовий пап≥рець крапл≥ досл≥джуваного розчину пап≥рець стаЇ червоним, то реакц≥€ роз≠чину кисла (рЌ < 5). ѕосин≥нн€ червоного лакмусового пап≥рц€ в≥д крапл≥ досл≥джуваного розчину показуЇ, що розчин маЇ луж≠ну реакц≥ю (рЌ > 8).
якщо н≥ рожевий, н≥ син≥й лакмусов≥ пап≥рц≥ не зм≥нюють свого кольору, то досл≥джуваний розчин нейтральний (рЌЂ7).
| Ќин≥ дл€ визначенн€ рЌ розчину часто користуютьс€ ун≥вер≠сальними ≥ндикаторами або ун≥версальним ≥ндикаторним папе≠ром. ”н≥версальними ≥ндикаторами називаютьс€ сум≥ш≥ з к≥лькох простих ≥ндикатор≥в, за допомогою €ких можна визначити вели≠чину рЌ у досить широкому ≥нтервал≥ значень з точн≥стю до 1-2. “ак Ї ун≥версальний ≥ндикатор ольтгофа, €кий складаЇтьс€ з п'€ти р≥зних ≥ндикатор≥в (фенолфталењн, метиловий червоний, бромтимоловий син≥й, тимолфгалењн, диметилам≥ноазобензен) ≥ зм≥нюЇ св≥й кол≥р у ≥нтервал≥ рЌ 2-10. ”н≥версальний ≥ндикатор використовуЇтьс€ у вигл€д≥ розчи≠ну (крапл€ ≥ндикатора зм≥шуЇтьс€ на краплинн≥й пластинц≥ з 1-2 крапл€ми досл≥джуваного розчину) або у вигл€д≥ ≥ндикатор≠ного паперу, €кий просочений розчином ун≥версального ≥ндика≠тора ≥ висушений. ƒо пачки ≥ндикаторного паперу додаЇтьс€ кольорова шкала, €ка показуЇ, €кого кольору набуваЇ ≥ндикаторний пап≥р при р≥зних величинах рЌ. ¬изначенн€ рЌ за шкалою за допомогою ≥ндикаторного пап≥рц€ значно спрощуЇ ≥ прискорюЇ процес виз≠наченн€ рЌ ≥ даЇ досить точн≥ результати. |
ƒл€ визначенн€ рЌ середовища зручно користуватис€ жов≠тим ун≥версальним папером, €кий просочений розчинами чоти≠рьох ≥ндикатор≥в (фенолфталењну, метилового червоного, бром- метилового синього, тимолового синього). Ќижче показано за≠барвленн€ жовтого ун≥версального ≥ндикатора залежно в≥д вели≠чини рЌ
Ѕ≥лет 22
1. ƒл€ розчину одноосновноњ сильноњ кислоти концентрац≥ю йон≥в г≥дрогену та њњ кислотн≥сть розраховують за р≥вн€нн€ми
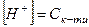 ,
, 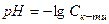 , (2.14)
, (2.14)
де 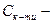 мол€рна концентрац≥€ одноосновноњ сильноњ кислоти, моль/л.
мол€рна концентрац≥€ одноосновноњ сильноњ кислоти, моль/л.
ƒл€ сильних однокислотних основ концентрац≥€ йон≥в  визначаЇтьс€ за р≥вн€нн€ми
визначаЇтьс€ за р≥вн€нн€ми
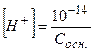 ;
; 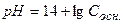 ,
,
рќЌ = -lg—м осн рЌ = 14 Ц рќЌ
2. —(¬а—l2)= 0,001 M
|
|
|
C (Na2CO3) = 0,001 M
як≥ мол€рн≥ концентрац≥њ кожноњ речовини у розчин≥ п≥сл€ зм≥шуванн€:
—(¬а—l2)= C (Na2CO3) = 0,0005 моль/дм3
як≥ мол€рн≥ концентрац≥њ ¬а 2+ ≥ —ќ2 3- п≥сл€ зм≥шуванн€
¬а—l2 = ¬а 2+ + 2—l- Na2CO3 = 2Na+ + —ќ2 3-
0,0005 1 моль
1 моль 0,0005
ƒобуток мол€рних концентрац≥й:
[¬а 2+][ —ќ2 3-]= …д = 0,0005. 0,0005 = 2,5 * 10-7
2,5 * 10-7 < 5 * 10-7 Ц осад не утворитьс€
≈кзаменац≥йний б≥лет є23






